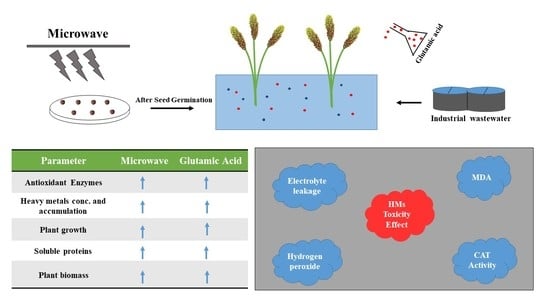
Microwave Irradiation and Glutamic Acid-Assisted Phytotreatment of Textile and Surgical Industrial Wastewater by Sorghum
Abstract
:1. Introduction
2. Results
2.1. Seed Germination Potential under Microwave Radiation
2.2. Morphological Characteristics
2.3. Biochemical Characteristics
2.4. Soluble Protein and SPAD Value
2.5. ROS Content in Sorghum
2.6. Response of Antioxidants in Sorghum
2.7. Heavy Metals Concentration and Accumulation in Sorghum
3. Discussion
4. Materials and Methods
4.1. Growth Conditions and Experimental Setup
4.2. Analysis of Morpho-Physiological Response in Plants
4.3. Determination of Antioxidant Enzymes, SPAD Value, and Soluble Protein Content
4.4. Evaluation of Electrolyte Leakage (EL), Malondialdehyde (MDA), and Hydrogen Peroxide H2O2
4.5. Assessment of Heavy Metals Concentration
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ashraf, S.; Afzal, M.; Rehman, K.; Naveed, M.; Zahi, Z.A. Plant endophyte synergism in constructed wetlands enhances the remediation of tannery effluent. Water Sci. Technol. 2018, 77, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Dustgeer, M.R.; Asma, S.T.; Jilani, A.; Raza, K.; Hussain, S.Z.; Shakoor, M.B.; Iqbal, J.; Abdel-wahab, M.S.; Darwesh, R. Synthesis and characterization of a novel single-phase sputtered Cu2O thin films: Structural, antibacterial activity and photocatalytic degradation of methylene blue. Inorg. Chem. Commun. 2021, 128, 108606. [Google Scholar] [CrossRef]
- Naeem, N.; Khalid, N.; Sarfraz, W.; Ejaz, U.; Yousaf, A.; Rizvi, Z.F.; Ikram, S. Assessment of Lead and Cadmium Pollution in Soil and Wild Plants at Different Functional Areas of Sialkot. Bull. Environ. Contam. Toxicol. 2021, 107, 336–342. [Google Scholar] [CrossRef]
- Jilani, A.; Hussain, S.Z.; Ansari, M.O.; Kumar, R.; Dustgeer, M.R.; Othman, M.H.D.; Barakat, M.A.; Melaibari, A.A. Facile synthesis of silver decorated reduced graphene oxide@ zinc oxide as ternary nanocomposite: An efficient photocatalyst for the enhanced degradation of organic dye under UV–visible light. J. Mater. Sci. 2021, 56, 7434–7450. [Google Scholar] [CrossRef]
- Khalid, N.; Rizvi, Z.F.; Yousaf, N.; Khan, S.M.; Ali, N.; Aqeel, M.; Latif, K.; Rafique, A. Rising Metals Concentration in the Environment: A Response to Effluents of Leather Industries in Sialkot. Bull. Environ. Contam. Toxicol. 2021, 106, 493–500. [Google Scholar] [CrossRef]
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chatta, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J.T. An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms and amelioration through selenium supplementation. Int. J. Mol. Sci. 2019, 21, 148. [Google Scholar] [CrossRef]
- Ullah, A.; Rahman, L.; Hussain, S.Z.; Abbas, W.; Tawab, A.; Jilani, A.; Bajwa, S.Z.; Khan, W.S.; Riaz, R.; Hussain, I.; et al. Mechanistic insight of dye degradation using TiO2 anchored α-MnO2 nanorods as promising sunlight driven photocatalyst. Mater. Sci. Eng. B 2021, 271, 115257. [Google Scholar] [CrossRef]
- Vareda, J.P.; Valente, A.J.; Duraes, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef]
- Ayadi, I.; Souissi, Y.; Jlassi, I.; Peixoto, F.; Mnif, W. Chemical synonyms, molecular structure and toxicological risk assessment of synthetic textile dyes: A critical review. J. Dev. Drugs 2016, 5, 2. [Google Scholar] [CrossRef]
- Farid, M.; Muntaha, S.; Abubakar, M.; Farid, S.; Sarfraz, W.; Ali, S.; Asam, Z.U.Z.; Zubair, M.; Rizwan, M. Efficacy of Spinach (Spinacia oleracea) for the Phyto-Management of Different Heavy Metals Contaminated Sites Under Chelating Agent Amendments. A Review. In Managing Plant Production Under Changing Environment; Hasanuzzaman, M., Ahammed, G.J., Nahar, K., Eds.; Springer: Singapore, 2022; pp. 293–310. [Google Scholar] [CrossRef]
- Yousaf, A.; Khalid, N.; Aqeel, M.; Noman, A.; Naeem, N.; Sarfraz, W.; Ejaz, U.; Qaiser, Z.; Khalid, A. Nitrogen Dynamics in Wetland Systems and Its Impact on Biodiversity. Nitrogen 2021, 2, 196–217. [Google Scholar] [CrossRef]
- Khlifi, R.; Sternberg, S.P.; Claussen, K. Lead and nickel removal using Microspora and Lemna minor. Bioresour. Technol. 2010, 89, 41–48. [Google Scholar]
- Jilani, A.; Othman, M.H.D.; Ansari, M.O.; Oves, M.; Hussain, S.Z.; Khan, I.U.; Abdel-Wahab, M.S. Structural and optical characteristics, and bacterial decolonization studies on non-reactive RF sputtered Cu–ZnO@ graphene based nanoparticles thin films. J. Mater. Sci. 2019, 54, 6515–6529. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Zubair, M.; Saeed, R.; Rizwan, M.; Sallah-Ud-Din, R.; Azam, A.; Ashraf, R.; Ashraf, W. Glutamic acid assisted phyto-management of silver contaminated soils through sunflower; physiological and biochemical response. Environ. Sci. Pollut. Res. 2018, 25, 25390–25400. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, D. Role of low cost agro-based adsorbent to treat hospital wastewater. Pollut. Res. 2014, 33, 573–576. [Google Scholar]
- Afshan, S.; Ali, S.; Bharwana, S.A.; Rizwan, M.; Farid, M.; Abbas, F.; Ibrahim, M.; Mehmood, M.A.; Abbasi, G.H. Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica L. Environ. Sci. Pollut. 2015, 22, 11679–11689. [Google Scholar] [CrossRef]
- Muratova, A.; Lyubun, Y.; German, K.; Turkovskaya, O. Effect of cadmium stress and inoculation with a heavy-metal-resistant bacterium on the growth and enzyme activity of Sorghum bicolor. Environ. Sci. Pollut. Res. 2015, 22, 16098–16109. [Google Scholar] [CrossRef]
- Feng, J.; Jia, W.; Lv, S.; Bao, H.; Miao, F.; Zhang, X.; Wang, J.; Li, J.; Li, D.; Zhu, C.; et al. Comparative transcriptome combined with morpho-physiological analyses revealed key factors for differential cadmium accumulation in two contrasting sweet sorghum genotypes. Plant Biotechnol. J. 2018, 16, 558–571. [Google Scholar] [CrossRef]
- He, S.; Yang, X.; He, Z.; Baligar, V.C. Morphological and physiological responses of plants to cadmium toxicity: A review. Pedosphere 2017, 27, 421–438. [Google Scholar] [CrossRef]
- Meyer, C.L.; Juraniec, M.; Huguet, S.; Chaves-Rodriguez, E.; Salis, P.; Isaure, M.P.; Goormaghtigh, E.; Verbruggen, N. Intraspecific variability of cadmium tolerance and accumulation, and cadmium-induced cell wall modifications in the metal hyperaccumulator Arabidopsis halleri. J. Exp. Bot. 2015, 66, 3215–3227. [Google Scholar] [CrossRef]
- Li, G.; Peng, X.; Xuan, H.; Wei, L.; Yang, Y.; Guo, T.; Kang, G. Proteomic analysis of leaves and roots of common wheat (Triticum aestivum L.) under copper-stress conditions. J. Proteome Res. 2013, 12, 4846–4861. [Google Scholar] [CrossRef]
- Roy, S.K.; Cho, S.W.; Kwon, S.J.; Kamal, A.H.M.; Lee, D.G.; Sarker, K.; Lee, M.S.; Xin, Z.; Woo, S.H. Proteome characterization of copper stress responses in the roots of sorghum. Biometals 2017, 30, 765–785. [Google Scholar] [CrossRef] [PubMed]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead uptake, toxicity, and detoxification in plants. Rev. Environ. Contam. Toxicol. 2011, 213, 113–136. [Google Scholar] [PubMed]
- Krzeslowska, M.; Rabeda, I.; Basinska, A.; Lewandowski, M.; Mellerowicz, E.J.; Napieralska, A.; Samardakiewicz, S.; Wozny, A. Pectinous cell wall thickenings formation—A common defense strategy of plants to cope with Pb. Environ. Pollut. 2016, 214, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.; Farid, S.; Zubair, M.; Rizwan, M.; Ishaq, H.; Ali, S.; Ashraf, U.; Alhaithloul, H.; Gowayed, S.; Soliman, M. Efficacy of Zea mays L. for the management of marble effluent contaminated soil under Citric acid amendment; morpho-physiological and biochemical response. Chemosphere 2020, 240, 124930. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, H.; Farid, M.; Zubair, M.; Alharby, H.; Asam, Z.U.Z.; Farid, S.; Bamagoos, A.; Alharbi, B.; Shakoor, M.; Ahmad, S.; et al. Efficacy of Lemna minor and Typha latifolia for the treatment of textile industry wastewater in a constructed wetland under citric acid amendment: A lab scale study. Chemosphere 2021, 283, 131107. [Google Scholar] [CrossRef]
- Abou-Elwafa, S.F. Identification of genes associated with drought tolerance in barley. Biol. Plant. 2018, 62, 299–306. [Google Scholar] [CrossRef]
- Abou-Elwafa, S.F.; Shehzad, T. Genetic identification and expression profiling of drought responsive genes in sorghum. Environ. Exp. Bot. 2018, 155, 12–20. [Google Scholar] [CrossRef]
- Zaheer, I.; Ali, S.; Saleem, M.H.; Imran, M.; Alnusairi, G.; Alharbi, B.; Riaz, M.; Abbas, Z.; Rizwan, M.; Soliman, M. Role of iron-lysine on morpho-physiological traits and combating chromium toxicity in rapeseed (Brassica napus L.) plants irrigated with different levels of tannery wastewater. Plant Physiol. Biochem. 2021, 155, 70–84. [Google Scholar] [CrossRef]
- Bashir, A.; Rizwan, M.; Ali, S.; Rehman, M.Z.; Ishaque, W.; Riaz, M.A.; Mabool, A. Effect of foliar-applied iron complexed with lysine on growth and cadmium (Cd) uptake in rice under Cd stress. Environ. Sci. Pollut. Res. 2018, 25, 20691–20699. [Google Scholar] [CrossRef]
- Noroozlo, Y.A.; Souri, M.K.; Delshed, M. Stimulation Effects of Foliar Applied Glycine and Glutamine Amino Acids on Lettuce Growth. Open Agric. 2019, 4, 164–172. [Google Scholar] [CrossRef]
- Guo, D.; Ali, A.; Ren, C.; Li, R.; Lahori, A.H.; Xiao, R.; Zhang, Z.; Zhang, Z. EDTA and organic acids assisted phytoextraction of Cd and Zn from a smelter contaminated soil by potherb mustard (Brassica juncea, Coss) and evaluation of its bioindicators. Ecotoxicol. Environ. Saf. 2019, 167, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Barros-Galvao, T.; Oliveira, D.F.A.; Macedo, C.E.C.; Voigt, E.L. Modulation of reserve mobilization by sucrose, glutamine, and abscisic acid during seedling establishment in sunflower. J. Plant Growth Regul. 2017, 36, 11–21. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, S.; Rizwan, M.; ur Rehman, M.Z.; Hameed, A.; Hafeez, F.; Wijaya, L. Role of zinc–lysine on growth and chromium uptake in rice plants under Cr stress. J. Plant Growth Regul. 2018, 37, 1413–1422. [Google Scholar] [CrossRef]
- Souri, M.K.; Hatamian, M. Aminochelates in plant nutrition: A review. J. Plant Nutr. 2019, 42, 67–78. [Google Scholar] [CrossRef]
- Sadak, M.; Abdoelhamid, M.T.; Schmidhalter, U. Effect of foliar application of amino acids on plant yield and some physiological parameters in bean plants irrigated with sea water. Acta Biol. Colomb. 2015, 20, 141–152. [Google Scholar]
- Souri, M.K.; Naiji, M.; Aslani, M. Effect of Fe-glycine aminochelate on pod quality and iron concentrations of bean (Phaseolus vulgaris L.) under lime soil conditions. Commun. Soil Sci. Plant Anal. 2018, 49, 215–224. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adress, M.; Ibrahim, M.; Zia-ur-Reham, M.; Farid, M.; Abbas, F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Hazard. Mater. 2017, 322, 2–16. [Google Scholar] [CrossRef]
- Jilani, A.; Ansari, M.O.; Rehman, G.U.; Shakoor, M.B.; Hussain, S.Z.; Othman, M.H.D.; Ahmad, S.R.; Dustgeer, M.R.; Alshahrie, A. Phenol removal and hydrogen production from water: Silver nanoparticles decorated on polyaniline wrapped zinc oxide nanorods. J. Ind. Eng. Chem. 2022, 109, 347–358. [Google Scholar] [CrossRef]
- Abu-Elsaoud, A.M.; Qari, S.H. Influence of Microwave Irradiations on Germination, Seedling Growth and Electrolyte Leakage of Barley (Hordeum vulgare L.). Int. J. Environ. Sci. 2017, 16, 11–24. [Google Scholar] [CrossRef]
- Abbey, L.; Udenigwe, C.; Mohan, A.; Anom, E. Microwave irradiation effects on vermicasts potency, and plant growth and antioxidant activity in seedlings of Chinese cabbage (Brassica rapa subsp. pekinensis). J. Radiat. Res. Appl. Sci. 2017, 10, 110–116. [Google Scholar] [CrossRef]
- Kutrowska, A.; Małecka, A.; Piechalak, A.; Masiakowski, W.; Hanć, A.; Barałkiewicz, D.; Andrzejewska, B.; Zbierska, J.; Tomaszewska, B. Effects of binary metal combinations on zinc, copper, cadmium and lead uptake and distribution in Brassica juncea. J. Trace Elem. Med. Biol. 2017, 44, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhou, Y.; Shen, Q.; Zhang, F. Effect of ammonium and nitrate nutrition on some physiological processes in higher plants-growth, photosynthesis, photorespiration, and water relations. Plant Biol. 2007, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Dalyan, E.; Yüzbaşıoğlu, E.; Akpınar, I. Physiological and Biochemical Changes in Plant Growth and Different Plant Enzymes in Response to Lead Stress. In Lead in Plants and the Environment; Springer: Cham, Switzerland, 2020; pp. 129–147. [Google Scholar]
- Jaiswal, A.; Verma, A.; Jaiswal, P. Detrimental effects of heavy metals in soil, plants, and aquatic ecosystems and in humans. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.J.; Raza, M.A.; Ur Rehman, S.; Ansar, M.; Gitari, H.; Khan, I.; Wajid, M.; Ahmed, M.; Shah, G.A.; Peng, Y.; et al. Effect of Cadmium Toxicity on Growth, Oxidative Damage, Antioxidant Defense System and Cadmium Accumulation in Two Sorghum Cultivars. Plants 2020, 9, 1575. [Google Scholar] [CrossRef]
- Xu, Z.; Ma, J.; Lei, P.; Wang, Q.; Feng, X.; Xu, H. Poly-γ-glutamic acid induces system tolerance to drought stress by promoting abscisic acid accumulation in Brassica napus L. Sci. Rep. 2020, 10, 252. [Google Scholar] [CrossRef]
- Manickavasagan, A.; Jayas, D.S.; White, N.D.G. Germination of wheat grains from uneven microwave heating in an industrial microwave dryer. Can. Biosyst. Eng. 2007, 49, 3. [Google Scholar]
- Hodzic, Z.; Pasalic, H.; Memisevic, A.; Scrabovic, M.; Saletovic, M.; Poljakovic, M. The influence of total phenols content on antioxidant capacity in the whole grain extracts. Eur. J. Sci. Res. 2009, 28, 471–477. [Google Scholar]
- Zhang, H.; Xu, Z.; Guo, K.; Huo, Y.; He, G.; Sun, H.; Sun, G. Toxic effects of heavy metal Cd and Zn on chlorophyll, carotenoid metabolism and photosynthetic function in tobacco leaves revealed by physiological and proteomics analysis. Ecotoxicol. Environ. Saf. 2020, 202, 110856. [Google Scholar] [CrossRef]
- Parvin, K.; Hasanuzzaman, M.; Bhuyan, M.H.M.; Mohsin, S.M.; Fujita, M. Quercetin mediated salt tolerance in tomato through the enhancement of plant antioxidant defense and glyoxalase systems. Plants 2019, 8, 247. [Google Scholar] [CrossRef]
- Khan, A.S.; Ahmad, B.; Jaskan, M.J.; Ahmad, R.; Malik, A.U. Foliar application of mixture of amino acids and seaweed (Ascophylum nodosum) extract improve growth and physicochemical properties of grapes. Int. J. Agric. Biol. 2012, 14, 383–388. [Google Scholar]
- Aladjadjiyan, A. Influence of microwave irradiation on some vitality indices and electroconductivity of ornamental perennial crops. J. Cent. Eur. Agric. 2002, 3, 4. [Google Scholar]
- Jakubowski, T. The impact of microwave radiation at different frequencies on weight of seed potato germs and crop of potato tubers. Inżynieria Rol. 2010, 14, 57–64. [Google Scholar]
- Basanth, N.; Mahesh, G. Bioefficacy of Nova Nutri Boost for yield and yield components in paddy (Oryza sativa L.). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2250–2253. [Google Scholar] [CrossRef]
- Souri, M.K.; Yaghoubi Sooraki, F.; Moghadamyar, M. Growth and quality of cucumber, tomato, and green bean plants under foliar and soil applications of an aminochelate fertilizer. Hortic. Environ. Biotechnol. 2017, 58, 530–536. [Google Scholar] [CrossRef]
- Ehsan, S.; Ali, S.; Noureen, S.; Mahmood, K.; Farid, M.; Ishaque, W.; Rizwan, M. Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicol. Environ. Saf. 2014, 106, 164–172. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Abbas, F.; Adrees, M.; Zia-ur-Rehman, M.; Farid, M.; Ali, B. Role of organic and inorganic amendments in alleviating heavy metal stress in oil seed crops. Oil Seed Crops Yield Adapt. Environ. Stress 2017, 12, 224–235. [Google Scholar]
- Farid, M.; Ali, S.; Rizwan, M.; Ali, Q.; Saeed, R.; Nasir, T.; Ahmad, T. Phyto-management of chromium contaminated soils through sunflower under exogenously applied 5-aminolevulinic acid. Ecotoxicol. Environ. Saf. 2018, 151, 255–265. [Google Scholar] [CrossRef]
- Etesami, H. Bacterial mediated alleviation of heavy metal stress and decreased accumulation of metals in plant tissues: Mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 147, 175–191. [Google Scholar] [CrossRef]
- Jaison, S.; Muthukumar, T. Chromium accumulation in medicinal plants growing naturally on tannery contaminated and non-contaminated soils. Biol. Trace Elem. Res. 2017, 175, 223–235. [Google Scholar] [CrossRef]
- Dordas, C.A.; Sioulas, C. Safflower yield, chlorophyll content, photosynthesis, and water use efficiency response to nitrogen fertilization under rainfed conditions. Ind. Crops Prod. 2008, 27, 75–85. [Google Scholar] [CrossRef]
- Mohammadipour, N.; Souri, M.K. Beneficial effects of glycine on growth and leaf nutrient concentrations of coriander (Coriandrum sativum) plants. J. Plant Nutr. 2019, 42, 1637–1644. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Rizwan, M.; Yasmeen, T.; Arif, M.S.; Riaz, M.; Saqib, M.; Zia urRehman, M.; Ayub, M.A. Combined effects of citric acid and 5-aminolevulinic acid in mitigating chromium toxicity in sunflower (Helianthus annuus L.) grown in Cr spiked soil. Pak. J. Agric. Sci. 2020, 57, 1–11. [Google Scholar]
- Khair, K.U.; Farid, M.; Ashraf, U.; Zubair, M.; Rizwan, M.; Farid, S.; Ali, S. Citric acid enhanced phytoextraction of nickel (Ni) and alleviate Mentha piperita (L.) from Ni-induced physiological and biochemical damages. Environ. Sci. Pollut. Res. 2020, 27, 27010–27022. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Lawrence, K.; Pandit, S.; Lawrence, R.S. Oxidative stress in leaves, stems and roots of Withania somnifera on copper exposure. Int. J. Pharm. Sci. Rev. Res. 2015, 30, 239–246. [Google Scholar]
- Souri, M.K. Amino chelate fertilizers: The new approach to the old problem; a review. Open Agric. 2016, 1, 118–123. [Google Scholar] [CrossRef]
- Sadak, M.; Abdelhamid, M. Influence of Amino Acids Mixture Application on Some Biochemical Aspects, Antioxidant Enzymes and Endogenous Polyamines of Vicia faba Plant Grown under Seawater Salinity Stress. Gesunde Pflanz. 2015, 67, 119–129. [Google Scholar] [CrossRef]
- Khalid, N.; Hussain, M.; Young, H.S.; Boyce, B.; Aqeel, M.; Noman, A. Effects of road proximity on heavy metal concentrations in soils and common roadside plants in Southern California. Environ. Sci. Pollut. Res. 2018, 25, 35257–35265. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Y.; Huang, Y.; Zeng, G.; Wang, Y.; Hu, X.; Zhou, L. Effects of indole-3-acetic, kinetin and spermidine assisted with EDDS on metal accumulation and tolerance mechanisms in ramie (Boehmeria nivea (L.) Gaud.). Ecol. Eng. 2014, 71, 108–112. [Google Scholar] [CrossRef]
- Rai, V.K. Role of amino acids in plant responses to stresses. Biol. Plant. 2002, 45, 481–487. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier: London, UK, 2011; p. 672. [Google Scholar]
- Stroppa, P.H.F.; Martins, J.S.; Dias, R.C.; Salla, C.A.; Bechtold, I.H.; Legnani, C.; da Silva, A.D. High efficient Light-Emitting Electrochemical Cells based on ionic liquids 1, 2, 3-triazolium. Org. Electron. 2019, 73, 172–181. [Google Scholar] [CrossRef]
- Duman, F.; Urey, E.; Koca, F.D. Temporal variation of heavy metal accumulation and translocation characteristics of narrow-leaved cattail (Typha angustifolia L.). Environ. Sci. Pollut. Res. 2015, 22, 17886–17896. [Google Scholar] [CrossRef] [PubMed]
- Klink, A. A comparison of trace metal bioaccumulation and distribution in Typha latifolia and Phragmites australis: Implication for phytoremediation. Environ. Sci. Pollut. Res. 2017, 24, 3843–3852. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+ permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010, 28, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Noman, A.; Ali, S.; Naheed, F.; Ali, Q.; Farid, M.; Rizwan, M.; Irshad, M.K. Foliar application of ascorbate enhances the physiological and biochemical attributes of maize (Zea mays L.) cultivars under drought stress. Arch. Agron. Soil Sci. 2015, 61, 1659–1672. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Ali, S.; Bharwana, S.A.; Rizwan, M.; Farid, M.; Kanwal, S.; Ali, Q.; Khan, M.D. Fulvic acid mediates chromium (Cr) tolerance in wheat (Triticum aestivum L.) through lowering of Cr uptake and improved antioxidant defense system. Environ. Sci. Pollut. Res. 2015, 22, 10601–10609. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Hussain, A.; Ali, Q.; Shakoor, M.B.; Zia-ur-Rehman, M.; Farid, M.; Asma, M. Effect of zinc-lysine on growth, yield and cadmium uptake in wheat (Triticum aestivum L.) and health risk assessment. Chemosphere 2017, 187, 35–42. [Google Scholar] [CrossRef]
- Sytar, O.; Kumari, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone priming: Regulator for heavy metal stress in plants. J. Plant Growth Regul. 2019, 38, 739–752. [Google Scholar] [CrossRef]
- Ullah, A.; Heng, S.; Munis, M.F.H.; Fahad, S.; Yang, X. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: A review. Environ. Exp. Bot. 2015, 117, 28–40. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Zeng, P.; Guo, Z.; Xiao, X.; Peng, C.; Huang, B.; Feng, W. Complementarity of co-planting a hyperaccumulator with three metal (loid)-tolerant species for metal (loid)-contaminated soil remediation. Ecotoxicol. Environ. Saf. 2019, 169, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Metzner, H.; Rau, H.; Senger, H. Untersuchungen zur synchronisierbarkeit einzelner pigmentmangel-mutanten von Chlorella. Planta 1965, 65, 186–194. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Aebi, H. Catalase in vitro methods. Enzymol 1984, 105, 121–126. [Google Scholar]
- Zhang, X.Z. (Ed.) The Measurement and Mechanism of Lipid Peroxidation and SOD POD and CAT Activities in Biological System. In Research Methodology of Crop Physiology; Beijing Agric Press: Beijing, China, 1992; pp. 208–211. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Zhang, J.; Kirkham, M.B. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol. 1996, 132, 361–373. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Rizwan, M.; Saeed, R.; Tauqeer, H.M.; Sallah-Ud-Din, R.; Azam, A.; Raza, N. Microwave irradiation and citric acid assisted seed germination and phytoextraction of nickel (Ni) by Brassica napus L.: Morpho-physiological and biochemical alterations under Ni stress. Environ. Sci. Pollut. Res. 2017, 24, 21050–21064. [Google Scholar] [CrossRef] [PubMed]





| Wastewater Conc. | Cd Concentration (µg g−1) | Cd Accumulation (µg Plant−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0% | 25% | 50% | 75% | 100% | 0% | 25% | 50% | 75% | 100% | |
| Treatments | Leaf | Leaf | ||||||||
| MR 0, G.A 0 | 0.00 ± 0.00 u | 136.81 ± 15.04 t | 438.33 ± 20.20 o | 757.67 ± 15.01 i | 1035 ± 8 d | 0.04 ± 0.02 q | 1256.29 ± 170.59 p | 3203 ± 255.37 mn | 3838.8 ± 113.00 lm | 3209.33 ± 128.30 mn |
| MR 30 | 0.00 ± 0.00 u | 171.66 ± 12.58 t | 497.66 ± 13.01 n | 812.67 ± 15.04 h | 1066 ± 7.94 cd | 0.07 ± 0.04 q | 1806.66 ± 218.30 op | 4065.1 ± 163.57 klm | 5121.8 ± 256.95 hij | 4442.36 ± 192.21 jkl |
| G.A 5 | 0.03 ± 0.02 u | 216.66 ± 15.27 s | 537.33 ± 12.50 m | 869.33 ± 18.00 g | 1092.33 ± 7.02 c | 0.48 ± 0.30 q | 2566.66 ± 238.60 no | 4872.66 ± 170.98 ijk | 6320.16 ± 348.77 efg | 5680.6 ± 145.62 ghi |
| G.A 10 | 0.05 ± 0.01 u | 266.67 ± 12.58 r | 583 ± 9.16 l | 917.33 ± 10.50 f | 1145.34 ± 15.01 b | 0.84 ± 0.22 q | 3442.83 ± 254.25 lmn | 6124.5 ± 385.81 fgh | 7462.033 ± 225.17 cd | 7064.4 ± 266.35 def |
| MR 30 + G.A 5 | 0.08 ± 0.00 u | 310.67 ± 16.01 q | 631.34 ± 15.04 k | 943.34 ± 8.08 f | 1195.66 ± 10.06 a | 1.38 ± 0.09 q | 4447.66 ± 120.60 jkl | 7150.33 ± 238.75 de | 8808 ± 560.63 b | 8768.66 ± 702.66 b |
| MR 30 + G.A 10 | 0.09 ± 0.00 u | 353.33 ± 13.50 p | 673.33 ± 13.50 j | 984.33 ± 13.01 e | 1231.33 ± 15.04 a | 1.71 ± 0.09 q | 5413.33 ± 107.50 ghij | 8304.66 ± 800.34 bc | 10167 ± 449.16 a | 10256.33 ± 607.15 a |
| Stem | Stem | |||||||||
| MR 0, G.A 0 | 0.00 ± 0.00 v | 160 ± 20 u | 623.33 ± 17.56 o | 1036.67 ± 20.55 j | 1369.33 ± 10.06 e | 0.07 ± 0.02 q | 2346.66 ± 313.90 p | 8207.5 ± 302.37 lm | 12482.1 ± 819.41 ij | 13512 ± 399.43 ghi |
| MR 30 | 0.00 ± 0.00 v | 230 ± 25 t | 683.67 ± 16.04 n | 1080.33 ± 15.50 ij | 1415.67 ± 15.04 de | 0.11 ± 0.03 q | 3825 ± 306.47 op | 9685.5 ± 309.73 kl | 14399.33 ± 454.31 gh | 16515.67 ± 824.14 ef |
| G.A 5 | 0.02 ± 0.01 v | 297.66 ± 20.40 s | 743 ± 18.08 m | 1134.67 ± 20.40 hi | 1467.66 ± 11.71 cd | 0.39 ± 0.18 q | 5402 ± 324.22 no | 11070.5 ± 288.99 jk | 17251.4 ± 697.76 e | 19372.8 ± 1054.41 d |
| G.A 10 | 0.05 ± 0.01 v | 361.67 ± 20.21 r | 783.66 ± 13.05 m | 1186 ± 6.55 gh | 1523.33 ± 21.73 bc | 1.16 ± 0.29 q | 6932.5 ± 412.39 mn | 12695.67 ± 357.21 hij | 19610.67 ± 702.446 d | 21826.67 ± 593.78 c |
| MR 30 + G.A 5 | 0.07 ± 0.01 v | 433.33 ± 30.13 q | 845.33 ± 17.89 l | 1235.33 ± 11.06 fg | 1573 ± 11.78 b | 1.17 ± 0.30 q | 8879.16 ± 567.79 l | 14799.17 ± 733.41 fg | 22651.67 ± 905.45 c | 24851.4 ± 429.32 b |
| MR 30 + G.A 10 | 0.09 ± 0.01 v | 524.66 ± 30.27 p | 920.66 ± 22.72 k | 1277.33 ± 9.29 f | 1650 ± 32.78 a | 2.20 ± 0.08 q | 11473.33 ± 692.30 jk | 17070.4 ± 877.58 e | 25118.33 ± 622.82 b | 28598.33 ± 1035.78 a |
| Root | Root | |||||||||
| MR 0, G.A 0 | 0.02 ± 0.01 r | 293.76 ± 11.93 q | 910 ± 20 m | 1645 ± 15 h | 2055 ± 18.02 de | 0.169 ± 0.08 m | 2146.49 ± 166.45 l | 5613 ± 237 ij | 6911 ± 392.00 fghi | 6506.16 ± 181.94 hi |
| MR 30 | 0.03 ± 0.01 r | 380 ± 20 pq | 1000 ± 18.02 m | 1731.67 ± 30.14 h | 2136.67 ± 25.16 cd | 0.281 ± 0.09 m | 3118.66 ± 240.01 kl | 6735.83 ± 327.96 ghi | 8316 ± 490.11 ef | 7477.33 ± 175.28 fgh |
| G.A 5 | 0.06 ± 0.01 r | 463.33 ± 25.16 p | 1136.67 ± 50.33 l | 1838.33 ± 37.52 g | 2210 ± 30 bc | 0.69 ± 0.17 m | 4297.66 ± 347.91 jk | 8268 ± 647.70 efg | 10053.33 ± 483.45 cd | 9058 ± 285.88 de |
| G.A 10 | 0.07 ± 0.01 r | 566.67 ± 27.53 o | 1326.67 ± 25.16 k | 1888.33 ± 36.17 fg | 2295 ± 35 b | 0.89 ± 0.20 m | 5593.83 ± 356.00 ij | 10529.67 ± 612.51 cd | 11327.83 ± 93.53 c | 10784.17 ± 405.35 c |
| MR 30 + G.A 5 | 0.08 ± 0.01 r | 635 ± 18.02 no | 1436 ± 67 ± 45.09 j | 1965 ± 25 ef | 2398.33 ± 50.08 a | 1.02 ± 0.13 m | 6766.66 ± 195.53 ghi | 13467.33 ± 924.91 b | 13624 ± 286.54 b | 13664 ± 466.26 b |
| MR 30 + G.A 10 | 0.09 ± 0.01 r | 725 ± 40.92 n | 1538.33 ± 25.65 i | 1991.67 ± 24.66 e | 2473.34 ± 76.53 a | 1.23 ± 0.18 m | 8275.16 ± 103.39 efg | 16144.17 ± 509.53 a | 15735.83 ± 484.71 a | 16100.83 ± 1724.46 a |
| Wastewater Conc. | Cu Concentration (µg g−1) | Cu Accumulation (µg Plant−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0% | 25% | 50% | 75% | 100% | 0% | 25% | 50% | 75% | 100% | |
| Treatments | Leaf | Leaf | ||||||||
| MR 0, G.A 0 | 0.00 ± 0.00 t | 107.66 ± 11.23 s | 259.67 ± 5.50 n | 407.66 ± 12.85 i | 634 ± 9.54 e | 0.03 ± 0.02 o | 985.33 ± 82.28 n | 1895.33 ± 70.99 jk | 2064.53 ± 20.70 klm | 1965.433 ± 71.86 klm |
| MR 30 | 0.00 ± 0.00 t | 133.67 ± 7.02 rs | 286.33 ± 12.22 mn | 446 ± 19.52 h | 660 ± 15.39 e | 0.07 ± 0.02 o | 1402.16 ± 65.10 mn | 2337.76 ± 86.62 jkl | 2808.6 ± 113.45 ghij | 2749.26 ± 91.84 ij |
| G.A 5 | 0.03 ± 0.00 t | 152.66 ± 11.06 qr | 310.66 ± 8.50 lm | 478.33 ± 6.43 gh | 716.66 ± 18.50 d | 0.48 ± 0.06 o | 1804.83 ± 98.61 lm | 2816.93 ± 95.17 ghij | 3475.6 ± 117.76 efg | 3726.06 ± 87.43 ef |
| G.A 10 | 0.05 ± 0.00 t | 173 ± 8 pq | 326.67 ± 5.86 kl | 500.34 ± 11.67 g | 780.33 ± 13.01 c | 0.84 ± 0.07 o | 2230.36 ± 74.67 jkl | 3429.66 ± 165.86 efgh | 4068.8 ± 87.67 de | 4811.23 ± 94.46 c |
| MR 30 + G.A 5 | 0.07 ± 0.01 t | 194.34 ± 10.26 op | 346 ± 7.93 jk | 536.66 ± 12.09 f | 829.66 ± 13.01 b | 1.16 ± 0.10 o | 2788.33 ± 242.92 hij | 3923.33 ± 268.61 ef | 5004.33 ± 196.72 c | 6080 ± 397.93 b |
| MR 30 + G.A 10 | 0.08 ± 0.01 t | 218.66 ± 1.52 o | 372.66 ± 7.02 j | 564.33 ± 10.26 f | 881.67 ± 15.01 a | 1.46 ± 0.18 o | 3353.33 ± 145.11 fghi | 4600.66 ± 500.38 cd | 5834.33 ± 411.86 b | 7352.33 ± 626.62 a |
| Stem | Stem | |||||||||
| MR 0, G.A 0 | 0.00 ± 0.00 t | 127.33 ± 11.67 s | 283 ± 8.18 n | 431 ± 17.08 i | 656.33 ± 12.22 e | 0.04 ± 0.04 p | 1868.33 ± 200.64 o | 3726.5 ± 147.63 lm | 5184.1 ± 252.73 ij | 6474.4 ± 101.00 g |
| MR 30 | 0.00 ± 0.00 t | 153.73 ± 7.12 rs | 305 ± 10.58 mn | 466.33 ± 19.00 h | 681.66 ± 13.31 e | 0.11 ± 0.03 p | 2562.46 ± 156.19 no | 4321.5 ± 196.36 kl | 6211.33 ± 123.78 gh | 7957.66 ± 539.62 ef |
| G.A 5 | 0.05 ± 0.01 t | 172.66 ± 11.06 qr | 331 ± 8.71 lm | 498.34 ± 6.42 g | 733 ± 14.42 d | 1.05 ± 0.31 p | 3142.16 ± 329.86 mn | 4931.5 ± 120.53 ijk | 7575.2 ± 226.81 f | 9668.66 ± 334.23 d |
| G.A 10 | 0.07 ± 0.01 t | 193 ± 8 pq | 347.66 ± 5.77 kl | 521 ± 11.13 g | 797 ± 8.71 c | 1.44 ± 0.21 p | 3697.83 ± 109.06 lm | 5630.86 ± 25.74 hi | 8611.2 ± 184.82 e | 11425.67 ± 545.23 c |
| MR 30 + G.A 5 | 0.08 ± 0.01 t | 214.33 ± 10.26 op | 365 ± 8.18 jk | 557.66 ± 11.37 f | 850.33 ± 13.01 b | 1.81 ± 0.23 p | 4397.16 ± 314.65 jkl | 6386.66 ± 195.67 gh | 10225 ± 424.81 d | 13435.13 ± 353.28 b |
| MR 30 + G.A 10 | 0.08 ± 0.00 t | 235.33 ± 5.68 o | 392.66 ± 7.02 j | 584.33 ± 10.26 f | 901.66 ± 15.01 a | 2.05 ± 0.12 p | 5146.8 ± 176.66 ij | 7275.86 ± 149.42 f | 11491 ± 354.51 c | 15624 ± 325.91 a |
| Root | Root | |||||||||
| MR 0, G.A 0 | 0.00 ± 0.00 u | 194.51 ± 7.16 t | 487.66 ± 13.65 o | 763.33 ± 17.55 j | 1029.33 ± 24.00 f | 0.03 ± 0.03 p | 1419.61 ± 70.07 o | 3006.53 ± 77.40l m | 3203.66 ± 79.41 klm | 3257.8 ± 50.75 klm |
| MR 30 | 0.03 ± 0.00 u | 242.66 ± 8.02 s | 531.33 ± 12.50 no | 790.34 ± 20.00 ij | 1139.33 ± 14.36 e | 0.06 ± 0.02 p | 1989.26 ± 55.33 no | 3576.96 ± 111.17 jkl | 3792.33 ± 141.66 ijkl | 3986.933 ± 81.83 ijk |
| G.A 5 | 0.05 ± 0.01 u | 291.77 ± 7.12 r | 565.34 ± 14.50 mn | 834.67 ± 19.29 hi | 1240.34 ± 3.05 d | 0.51 ± 0.11 p | 2703.99 ± 109.99 mn | 4105.66 ± 39.75 hij | 4562.033 ± 128.88 fghi | 5085.1 ± 205.76 f |
| G.A 10 | 0.06 ± 0.01 u | 342.33 ± 16.62 q | 610 ± 13.52l m | 877.33 ± 16.01 h | 1344.33 ± 21.36 c | 0.73 ± 0.14 p | 3376 ± 111.33 ljklm | 4839.06 ± 214.35 fgh | 5263.5 ± 95.00 ef | 6318.83 ± 304.45 d |
| MR 30 + G.A 5 | 0.07 ± 0.00 u | 385 ± 13.45 pq | 645.33 ± 18.17 l | 930.66 ± 18.00 g | 1439.67 ± 15.53 b | 0.93 ± 0.89 p | 4110.33 ± 337.3 ghij | 6043.23 ± 237.33 de | 6454 ± 230.78 d | 8205.9 ± 382.89 b |
| MR 30 + G.A 10 | 0.08 ± 0.00 u | 428.66 ± 16.92 p | 698.33 ± 8.32 k | 958.67 ± 21.22 g | 1535 ± 21.79 a | 1.14 ± 0.11 p | 4904 ± 362.67 fg | 7333.16 ± 382.17 c | 7575.83 ± 328.87 bc | 9984.16 ± 896.42 a |
| Wastewater Conc. | Pb Concentration (µg g−1) | Pb Accumulation (µg Plant−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0% | 25% | 50% | 75% | 100% | 0% | 25% | 50% | 75% | 100% | |
| Treatments | Leaf | Leaf | ||||||||
| MR 0, G.A 0 | 0.00 ± 0.00 t | 135.33 ± 10.40 s | 316.66 ± 28.43 no | 445.67 ± 12.05 jk | 656.33 ± 12.22 e | 0.03 ± 0.02 p | 1239.16 ± 73.18 o | 2308.83 ± 175.43 lmn | 2258.13 ± 83.02 lmn | 2034.36 ± 64.57 mno |
| MR 30 | 0.01 ± 0.00 t | 169 ± 13.11 rs | 344 ± 17.69 mn | 466.33 ± 19.00 ij | 761.66 ± 17.56 d | 0.13 ± 0.10 p | 1771.83 ± 106.86 no | 2808.83 ± 124.15 jklm | 2936.7 ± 111.31 jkl | 3172.33 ± 82.97 jk |
| G.A 5 | 0.04 ± 0.01 t | 186 ± 11.53 rs | 367.66 ± 19.00l mn | 501.67 ± 11.01 hi | 816 ± 26.51 c | 0.57 ± 0.13 p | 2199.16 ± 96.87 lmn | 3332.26 ± 138.43 ijk | 3644.26 ± 98.12 hij | 4242.03 ± 102.89 gh |
| G.A 10 | 0.05 ± 0.02 t | 213 ± 21.07 qr | 390.33 ± 3.05 lm | 527.66 ± 22.30 gh | 954.66 ± 27.97 b | 0.84 ± 0.32 p | 2742.7 ± 194.95 klm | 4098.16 ± 187.91 ghi | 4290.26 ± 143.24 fgh | 5886.033 ± 180.33 cd |
| MR 30 + G.A 5 | 0.08 ± 0.01 t | 253 ± 7.55 pq | 409 ± 10.58 kl | 561.33 ± 16.50 fg | 992.33 ± 14.36 b | 1.34 ± 0.25 p | 3628.66 ± 243.28 hij | 4638 ± 328.95 efg | 5233.66 ± 202.36 cde | 7271.66 ± 464.80 b |
| MR 30 + G.A 10 | 0.09 ± 0.00 t | 282.33 ± 16.16 op | 415.66 ± 16.16 jkl | 589.34 ± 15.17 f | 1048.34 ± 36.01 a | 1.58 ± 0.05 p | 4334 ± 391.84 fgh | 5128.33 ± 547.92 def | 6094.33 ± 476.07 c | 8748.33 ± 890 a |
| Stem | Stem | |||||||||
| MR 0, G.A 0 | 0.00 ± 0.00 v | 116.66 ± 15.27 u | 418.34 ± 20.21 p | 721.66 ± 17.03 k | 1000.67 ± 3.05 ef | 0.05 ± 0.03 r | 1710 ± 226.49 q | 5507.5 ± 272.70 mn | 8690.26 ± 601.52 ijk | 9873.6 ± 254.35 hi |
| MR 30 | 0.03 ± 0.02 v | 151.67 ± 12.58 tu | 477.67 ± 13.01 o | 778 ± 14.52 jk | 1031 ± 7.93 de | 0.58 ± 0.47 r | 2523.33 ± 132.03 pq | 6766.83 ± 223.43 lm | 10373.67 ± 498.06 h | 12029.33 ± 631.14 g |
| G.A 5 | 0.05 ± 0.01 v | 196.66 ± 15.27 st | 513.66 ± 7.67 no | 834.66 ± 18.50 ij | 1061 ± 10.14 cd | 0.98 ± 0.20 r | 3566.66 ± 189.03 op | 7653 ± 82.86 jkl | 12690.6 ± 552.34 fg | 14007.33 ± 830.28 ef |
| G.A 10 | 0.06 ± 0.01 v | 246.66 ± 12.58 rs | 564.33 ± 14.01 mn | 884.66 ± 7.63 hi | 1110 ± 15 bc | 1.24 ± 0.21 r | 4727.5 ± 241.41 no | 9142.33 ± 303.61 hij | 14628.93 ± 567.05 de | 15905 ± 462.41 cd |
| MR 30 + G.A 5 | 0.07 ± 0.00 v | 257.33 ± 44.23 r | 578.33 ± 60.92 m | 908.66 ± 8.08 gh | 1160 ± 10 ab | 1.74 ± 0.17 r | 5272.83 ± 891.51 mn | 10125.67 ± 1181.01 hi | 16661.33 ± 649.43 c | 18326 ± 278.47 b |
| MR 30 + G.A 10 | 0.09 ± 0.00 v | 333.33 ± 13.50 q | 653 ± 13 l | 950 ± 13.51 fg | 1199.33 ± 12.09 a | 2.20 ± 0.08 r | 7288.93 ± 308.44 kl | 12106.6 ± 568.01 g | 18679 ± 363.23 b | 20787 ± 655.94 a |
| Root | Root | |||||||||
| MR 0, G.A 0 | 0.00 ± 0.00 v | 141.66 ± 15.27 u | 443.33 ± 20.21 p | 763 ± 15 j | 1040 ± 8 e | 0.03 ± 0.01 q | 1037.16 ± 152.62 p | 2735 ± 169.55 lmn | 3206.6 ± 215.60 klm | 3293.33 ± 112.62 jklm |
| MR 30 | 0.04 ± 0.03 v | 176.67 ± 12.58 u | 503 ± 13 o | 818 ± 14.52 i | 1071 ± 7.93 de | 0.23 ± 0.29 q | 1450.33 ± 138.63 op | 3388.6 ± 189.70 jkl | 3928.33 ± 233.11 hij | 3748.1 ± 88.22 ijk |
| G.A 5 | 0.06 ± 0.01 v | 221.66 ± 15.27 t | 542.33 ± 12.50 n | 874.67 ± 18.50 h | 1101 ± 10.14 d | 0.44 ± 0.05 q | 2056.66 ± 196.21 no | 3943.03 ± 226.55 hij | 4783.36 ± 233.36 ef | 4515.2 ± 229.27 fgh |
| G.A 10 | 0.06 ± 0.01 v | 271.67 ± 12.58 s | 588 ± 9.16 m | 924.33 ± 8.08 g | 1150.33 ± 15.01 c | 0.69 ± 0.12 q | 2681.66 ± 164.17 mn | 4666.46 ± 254.30 fg | 5546.53 ± 140.69 d | 5408.56 ± 300.50 de |
| MR 30 + G.A 5 | 0.08 ± 0.00 v | 315.66 ± 16.01 r | 636.33 ± 15.04 l | 948.67 ± 8.08 g | 1200 ± 10 b | 1.06 ± 0.04 q | 3367.33 ± 257.68 jklm | 5963.8 ± 363.22 cd | 6577.33 ± 116.02 bc | 6839.66 ± 312.09 b |
| MR 30 + G.A 10 | 0.09 ± 0.00 v | 358.34 ± 13.50 q | 678.33 ± 13.50 k | 990 ± 13.52 f | 1238 ± 13.31 a | 1.27 ± 0.11 q | 4092.46 ± 47.30 ghi | 7118 ± 197.50 b | 7820.9 ± 196.96 a | 8047.833 ± 595.41 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farid, M.; Abubakar, M.; Asam, Z.U.Z.; Sarfraz, W.; Abbas, M.; Shakoor, M.B.; Ali, S.; Ahmad, S.R.; Jilani, A.; Iqbal, J.; et al. Microwave Irradiation and Glutamic Acid-Assisted Phytotreatment of Textile and Surgical Industrial Wastewater by Sorghum. Molecules 2022, 27, 4004. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27134004
Farid M, Abubakar M, Asam ZUZ, Sarfraz W, Abbas M, Shakoor MB, Ali S, Ahmad SR, Jilani A, Iqbal J, et al. Microwave Irradiation and Glutamic Acid-Assisted Phytotreatment of Textile and Surgical Industrial Wastewater by Sorghum. Molecules. 2022; 27(13):4004. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27134004
Chicago/Turabian StyleFarid, Mujahid, Muhammad Abubakar, Zaki Ul Zaman Asam, Wajiha Sarfraz, Mohsin Abbas, Muhammad Bilal Shakoor, Shafaqat Ali, Sajid Rashid Ahmad, Asim Jilani, Javed Iqbal, and et al. 2022. "Microwave Irradiation and Glutamic Acid-Assisted Phytotreatment of Textile and Surgical Industrial Wastewater by Sorghum" Molecules 27, no. 13: 4004. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27134004