Poly-(lactic-co-glycolic) Acid Nanoparticles Entrapping Pterostilbene for Targeting Aspergillus Section Nigri
Abstract
:1. Introduction
2. Results
2.1. NPs Preparation and Characterization
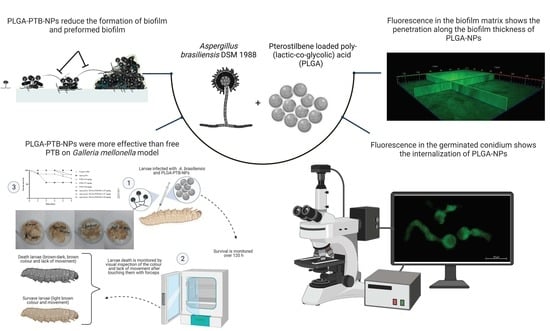
2.2. Microscopic Observations of NP Uptake in Aspergillus Conidia, Mycelium, and Biofilm
2.3. Antifungal Activity of PLGA-PTB-NPs
2.4. Activity of PLGA-PTB-NPs on a Model of Aspergillosis in G. mellonella
2.5. Toxicity on Galleria mellonella Larvae Model
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation and Characterization of PLGA-PTB-NPs and PLGA-coumarin6-NPs
4.3. Fungal Strain and Culture Condition
4.4. Fungal Uptake of PLGA-coumarin6-NPs
4.5. Microscopic Analysis
4.6. In Vitro Antifungal Activity of PLGA-PTB-NPs against Biofilm Formation and Preformed Biofilm
4.7. Toxicity of PLGA-PTB-NPs, Free PTB, and PLGA-NPs on Galleria mellonella Larvae Model
4.8. In Vivo Activity of PLGA-PTB-NPs
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Nucci, M.; Perfect, J.R. When Primary Antifungal Therapy Fails. Clin. Infect. Dis. 2008, 46, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Soliman, G.M. Nanoparticles as Safe and Effective Delivery Systems of Antifungal Agents: Achievements and Challenges. Int. J. Pharm. 2017, 523, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Nami, S.; Aghebati-Maleki, A.; Aghebati-Maleki, L. Current Applications and Prospects of Nanoparticles for Antifungal Drug Delivery. EXCLI J. 2021, 20, 562. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, S.; Manivannan, S.; Narayan, S. Potent Antifungal Agents and Use of Nanocarriers to Improve Delivery to the Infected Site: A Systematic Review. J. Basic Microbiol. 2021, 61, 849–873. [Google Scholar] [CrossRef] [PubMed]
- Osanloo, M.; Assadpour, S.; Mehravaran, A.; Abastabar, M.; Akhtari, J. Niosome-Loaded Antifungal Drugs as an Effective Nanocarrier System: A Mini Review. Curr. Med. Mycol. 2019, 4, 31–36. [Google Scholar] [CrossRef]
- Pardeshi, S.R.; Nikam, A.; Chandak, P.; Mandale, V.; Naik, J.B.; Giram, P.S. Recent Advances in PLGA Based Nanocarriers for Drug Delivery System: A State of the Art Review. Int. J. Polym. Mater. Polym. Biomater. 2021, 1, 1–30. [Google Scholar] [CrossRef]
- Dinarvand, R.; Sepehri, N.; Manouchehri, S.; Rouhani, H.; Atyabi, F. Polylactide-Co-Glycolide Nanoparticles for Controlled Delivery of Anticancer Agents. Int. J. Nanomed. 2011, 6, 877. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Skidmore, S.; Hadar, J.; Garner, J.; Park, H.; Otte, A.; Soh, B.K.; Yoon, G.; Yu, D.; Yun, Y.; et al. Injectable, Long-Acting PLGA Formulations: Analyzing PLGA and Understanding Microparticle Formation. J. Control. Release 2019, 304, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, G.; Palocci, C.; Valletta, A.; Kolesova, O.; Chronopoulou, L.; Donati, L.; Di Nitto, A.; Brasili, E.; Tomai, P.; Gentili, A.; et al. Anti-Candida Biofilm Activity of Pterostilbene or Crude Extract from Non-Fermented Grape Pomace Entrapped in Biopolymeric Nanoparticles. Molecules 2019, 24, 2070. [Google Scholar] [CrossRef]
- De Angelis, G.; Simonetti, G.; Chronopoulou, L.; Orekhova, A.; Badiali, C.; Petruccelli, V.; Portoghesi, F.; D’Angeli, S.; Brasili, E.; Pasqua, G.; et al. A Novel Approach to Control Botrytis Cinerea Fungal Infections: Uptake and Biological Activity of Antifungals Encapsulated in Nanoparticle Based Vectors. Sci. Rep. 2022, 12, 7989. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N. (Eds.) Molecular Biology of Food and Water Borne Mycotoxigenic and Mycotic Fungi; CRC Press: Boca Raton, FL, USA, 2015; ISBN 978-0-429-08764-6. [Google Scholar]
- Iatta, R.; Nuccio, F.; Immediato, D.; Mosca, A.; De Carlo, C.; Miragliotta, G.; Parisi, A.; Crescenzo, G.; Otranto, D.; Cafarchia, C. Species Distribution and In Vitro Azole Susceptibility of Aspergillus Section Nigri Isolates from Clinical and Environmental Settings. J. Clin. Microbiol. 2016, 54, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Kamali Sarvestani, H.; Seifi, A.; Falahatinejad, M.; Mahmoudi, S. Black Aspergilli as Causes of Otomycosis in the Era of Molecular Diagnostics, a Mini-Review. J. Med. Mycol. 2022, 32, 101240. [Google Scholar] [CrossRef] [PubMed]
- Bojanović, M.; Ignjatović, A.; Stalević, M.; Arsić-Arsenijević, V.; Ranđelović, M.; Gerginić, V.; Stojanović-Radić, Z.; Stojković, O.; Živković-Marinkov, E.; Otašević, S. Clinical Presentations, Cluster Analysis and Laboratory-Based Investigation of Aspergillus Otomycosis—A Single Center Experience. J. Fungi 2022, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.; Vrabec, J.T.; Yoo, D.; Coker, N.J. Otomycosis: Clinical Features and Treatment Implications. Otolaryngol. Neck Surg. 2006, 135, 787–791. [Google Scholar] [CrossRef]
- Gits-Muselli, M.; Hamane, S.; Verillaud, B.; Cherpin, E.; Denis, B.; Bondeelle, L.; Touratier, S.; Alanio, A.; Garcia-Hermoso, D.; Bretagne, S. Different Repartition of the Cryptic Species of Black Aspergilli According to the Anatomical Sites in Human Infections, in a French University Hospital. Med. Mycol. 2021, 59, 985–992. [Google Scholar] [CrossRef]
- Varga, J.; Kocsubé, S.; Tóth, B.; Frisvad, J.C.; Perrone, G.; Susca, A.; Meijer, M.; Samson, R.A. Aspergillus brasiliensis sp. Nov., a Biseriate Black Aspergillus Species with World-Wide Distribution. Int. J. Syst. Evol. Microbiol. 2007, 57, 1925–1932. [Google Scholar] [CrossRef]
- EN 14885:2015; Chemical Disinfectants and Antiseptics–Application of European Standards for Chemical Disinfectants and Antiseptics. iTeh, Inc.: Newark, NJ, USA, 2015.
- De Filippis, B.; Ammazzalorso, A.; Amoroso, R.; Giampietro, L. Stilbene Derivatives as New Perspective in Antifungal Medicinal Chemistry. Drug Dev. Res. 2019, 80, 285–293. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Ożarowski, M.; Seremak-Mrozikiewicz, A.; Wolski, H.; Adamczak, A. Plant Preparations and Compounds with Activities against Biofilms Formed by Candida spp. J. Fungi 2021, 7, 360. [Google Scholar] [CrossRef]
- Li, D.-D.; Zhao, L.-X.; Mylonakis, E.; Hu, G.-H.; Zou, Y.; Huang, T.-K.; Yan, L.; Wang, Y.; Jiang, Y.-Y. In Vitro and In Vivo Activities of Pterostilbene against Candida Albicans Biofilms. Antimicrob. Agents Chemother. 2014, 58, 2344–2355. [Google Scholar] [CrossRef]
- Smith, D.F.Q.; Casadevall, A. Fungal Immunity and Pathogenesis in Mammals versus the Invertebrate Model Organism Galleria mellonella. Pathog. Dis. 2021, 79, ftab013. [Google Scholar] [CrossRef]
- Li, Y.; Wan, Z.; Liu, W.; Li, R. Identification and Susceptibility of Aspergillus Section Nigri in China: Prevalence of Species and Paradoxical Growth in Response to Echinocandins. J. Clin. Microbiol. 2015, 53, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Toyotome, T.; Hagiwara, D.; Takahashi, H.; Watanabe, A.; Kamei, K. Emerging Antifungal Drug Resistance in Aspergillus Fumigatus and Among Other Species of Aspergillus. Curr. Fungal Infect. Rep. 2018, 12, 105–111. [Google Scholar] [CrossRef]
- Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Nanoparticle–Biofilm Interactions: The Role of the EPS Matrix. Trends Microbiol. 2019, 27, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.R.; Damann, K.; Leonardi, C.; Sabliov, C.M. Size Dependency of PLGA-Nanoparticle Uptake and Antifungal Activity against Aspergillus flavus. Nanomedicine 2011, 6, 1381–1395. [Google Scholar] [CrossRef]
- Muse, E.S.; Patel, N.R.; Astete, C.E.; Damann, K.E.; Sabliov, C.M. Surface Association and Uptake of Poly(Lactic-Co-Glycolic) Acid Nanoparticles by Aspergillus flavus. Ther. Deliv. 2014, 5, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Slomberg, D.L.; Lu, Y.; Broadnax, A.D.; Hunter, R.A.; Carpenter, A.W.; Schoenfisch, M.H. Role of Size and Shape on Biofilm Eradication for Nitric Oxide-Releasing Silica Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 9322–9329. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Domenici, F.; Giantulli, S.; Brasili, F.; D’Errico, C.; Tsaouli, G.; Tortorella, E.; Bordi, F.; Morrone, S.; Palocci, C.; et al. PLGA Based Particles as “Drug Reservoir” for Antitumor Drug Delivery: Characterization and Cytotoxicity Studies. Colloids Surf. B Biointerfaces 2019, 180, 495–502. [Google Scholar] [CrossRef]
- Fang, T.-H.; Kang, S.-H.; Hong, Z.-H.; Wu, C.-D. Elasticity and Nanomechanical Response of Aspergillus Niger Spores Using Atomic Force Microscopy. Micron 2012, 43, 407–411. [Google Scholar] [CrossRef]
- Durieux, M.-F.; Melloul, É.; Jemel, S.; Roisin, L.; Dardé, M.-L.; Guillot, J.; Dannaoui, É.; Botterel, F. Galleria mellonella as a Screening Tool to Study Virulence Factors of Aspergillus fumigatus. Virulence 2021, 12, 818–834. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Sparago, C.; Palocci, C. A Modular Microfluidic Platform for the Synthesis of Biopolymeric Nanoparticles Entrapping Organic Actives. J. Part Res. 2014, 16, 2703. [Google Scholar] [CrossRef]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A Simple and Reproducible 96-Well Plate-Based Method for the Formation of Fungal Biofilms and Its Application to Antifungal Susceptibility Testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, F.; D’Acierno, F.; Bortolami, M.; De Vita, D.; Gallo, F.; De Meo, A.; Di Santo, R.; Costi, R.; Simonetti, G.; Scipione, L. Searching for New Agents Active against Candida Albicans Biofilm: A Series of Indole Derivatives, Design, Synthesis and Biological Evaluation. Eur. J. Med. Chem. 2019, 165, 93–106. [Google Scholar] [CrossRef] [PubMed]





| Chemical | LD50 (mg/kg) | Solvent |
|---|---|---|
| PLGA-NPs | >2.85 | H2O |
|
PTB PLGA-PTB-NPs | >1.35 >2.85–1.35 |
100 H2O:1 DMSO 100 H2O:1 DMSO |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orekhova, A.; Palocci, C.; Chronopoulou, L.; De Angelis, G.; Badiali, C.; Petruccelli, V.; D’Angeli, S.; Pasqua, G.; Simonetti, G. Poly-(lactic-co-glycolic) Acid Nanoparticles Entrapping Pterostilbene for Targeting Aspergillus Section Nigri. Molecules 2022, 27, 5424. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27175424
Orekhova A, Palocci C, Chronopoulou L, De Angelis G, Badiali C, Petruccelli V, D’Angeli S, Pasqua G, Simonetti G. Poly-(lactic-co-glycolic) Acid Nanoparticles Entrapping Pterostilbene for Targeting Aspergillus Section Nigri. Molecules. 2022; 27(17):5424. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27175424
Chicago/Turabian StyleOrekhova, Anastasia, Cleofe Palocci, Laura Chronopoulou, Giulia De Angelis, Camilla Badiali, Valerio Petruccelli, Simone D’Angeli, Gabriella Pasqua, and Giovanna Simonetti. 2022. "Poly-(lactic-co-glycolic) Acid Nanoparticles Entrapping Pterostilbene for Targeting Aspergillus Section Nigri" Molecules 27, no. 17: 5424. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27175424