Nanocapsule of MnS Nanopolyhedron Core@CoS Nanoparticle/Carbon Shell@Pure Carbon Shell as Anode Material for High-Performance Lithium Storage
Abstract
:1. Introduction
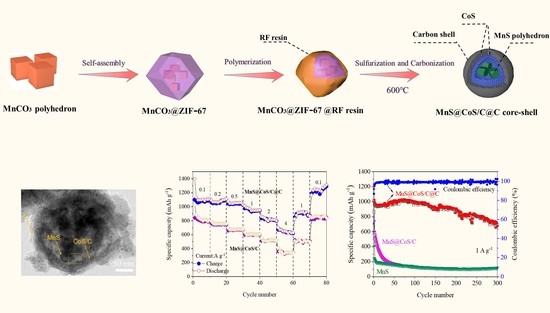
2. Results and Discussion
3. Experimental
3.1. Material Synthesis
3.2. Materials Characterizations
3.3. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhao, Z.; Xue, Z.; Xiong, Q.; Zhang, Y.; Hu, X.; Chi, H.; Qin, H.; Yuan, Y.; Ni, H. Titanium niobium oxides (TiNb2O7): Design, fabrication and application in energy storage devices. Sustain. Mater. Technol. 2021, 30, e00357. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Yuan, Y.F.; Zhu, M.; Yin, S.M.; Cheng, J.P.; Guo, S.Y. Superior rate-capability and long-lifespan carbon nanotube-in-nanotube@Sb2S3 anode for lithium-ion storage. J. Mater. Chem. A 2021, 9, 22334–22346. [Google Scholar] [CrossRef]
- Huang, X.; Guo, R.; Lin, Y.; Cao, Y.; Wu, J. Si/SiC/C in-situ composite microspindles as anode materials for lithium-ion batteries. Electrochim. Acta 2022, 422, 140546. [Google Scholar] [CrossRef]
- Chen, L.; Qiu, L.; Wang, H.; Yuan, Y.; Song, L.; Xie, F.; Xiong, J.; Du, P. CuGaO2 Nanosheet Arrays as the Hole-Transport Layer in Inverted Perovskite Solar Cells. ACS Appl. Nano Mater. 2022, 5, 10055–10063. [Google Scholar] [CrossRef]
- Qiu, L.; Mei, D.; Chen, W.-H.; Yuan, Y.; Song, L.; Chen, L.; Bai, B.; Du, P.; Xiong, J. Organic-inorganic hybrid electron transport layer of PVP-doped SnO2 for high-efficiency stable perovskite solar cells. Sol. Energy Mater. Sol. Cells 2022, 248, 112032. [Google Scholar] [CrossRef]
- Wang, B.; Gong, S.; Sun, Q.; Liu, F.; Wang, X.; Cheng, J. Carbon nanotubes refined mesoporous NiCoO2 nanoparticles for high−performance supercapacitors. Electrochim. Acta 2021, 402, 139575. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, Y.; Yao, Z.; Zhu, M.; Du, P.; Guo, S. CoCl2 encapsulated in nitrogen-doped carbon hollow cubic nanobox enabling long-life and high-rate lithium storage. Electrochim. Acta 2022, 430, 141092. [Google Scholar] [CrossRef]
- Yang, R.; Mei, L.; Fan, Y.; Zhang, Q.; Liao, H.-G.; Yang, J.; Li, J.; Zeng, Z. Fabrication of liquid cell for in situ transmission electron microscopy of electrochemical processes. Nat. Protoc. 2022. [Google Scholar] [CrossRef]
- Gong, S.; Wang, B.; Xue, Y.; Sun, Q.; Wang, J.; Kuai, J.; Liu, F.; Cheng, J. NiCoO2 and polypyrrole decorated three-dimensional carbon nanofiber network with coaxial cable-like structure for high-performance supercapacitors. J. Colloid Interface Sci. 2022, 628, 343–355. [Google Scholar] [CrossRef]
- Trukhanov, S.; Bodnar, I.; Zhafar, M. Magnetic and electrical properties of (FeIn2S4)1−x(CuIn5S8)x solid solutions. J. Magn. Magn. Mater. 2015, 379, 22–27. [Google Scholar] [CrossRef]
- Bodnar, I.; Jaafar, M.; Pauliukavets, S.; Trukhanov, S.; Victorov, I. Growth, optical, magnetic and electrical properties of CuFe2.33In9.67S17.33 single crystal. Mater. Res. Express 2015, 2, 085901. [Google Scholar] [CrossRef]
- Huang, L.; Guan, T.; Su, H.; Zhong, Y.; Cao, F.; Zhang, Y.; Xia, X.; Wang, X.; Bao, N.; Tu, J. Synergistic Interfacial Bonding in Reduced Graphene Oxide Fiber Cathodes Containing Polypyrrole@sulfur Nanospheres for Flexible Energy Storage. Angew. Chem. Int. Ed. 2022, 134, e202212151. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, R.; Li, H.; Zeng, Z. Boosting electrocatalytic reduction of CO2 to HCOOH on Ni single atom anchored WTe2 monolayer. Small 2022, 18, 2203759. [Google Scholar] [CrossRef]
- Liu, Y.; Su, H.; Zhong, Y.; Wang, X.; Xia, X.; Gu, C.; Tu, J. Revealing the Impact of Cl Substitution on the Crystallization Behavior and Interfacial Stability of Superionic Lithium Argyrodites. Adv. Funct. Mater. 2022, 32, 2207978. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, Y.; Zhu, M.; Yin, S.; Du, P.; Mo, C. Hierarchical hollow superstructure cobalt selenide bird nests for high-performance lithium storage. J. Colloid Interface Sci. 2022, 627, 449–458. [Google Scholar] [CrossRef]
- Wang, R.; Li, B.; Lai, L.; Hou, M.; Gao, J.; Wu, R. 3D urchin-like architectures assembled by MnS nanorods encapsulated in N-doped carbon tubes for superior lithium storage capability. Chem. Eng. J. 2018, 355, 752–759. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, M.; Wu, F.; Su, Z.; He, L.; Zhou, P.; Xu, P.; Zou, R.; Wang, X.; Huang, Q. Honeycomb-structured α-MnS@N-HC nanocomposite fabricated by sol-gel pyrolysis blowing method and its high-performance lithium storage. Mater. Today Energy 2021, 22, 100876. [Google Scholar] [CrossRef]
- Trukhanov, A.; Turchenko, V.; Bobrikov, I.; Trukhanov, S.; Kazakevich, I.; Balagurov, A. Crystal structure and magnetic properties of the BaFe12−xAlxO19 (x=0.1–1.2) solid solutions. J. Magn. Magn. Mater. 2015, 393, 253–259. [Google Scholar] [CrossRef]
- Zdorovets, M.; Kozlovskiy, A.; Shlimas, D.; Borgekov, D. Phase transformations in FeCo–Fe2CoO4/Co3O4-spinel nanostructures as a result of thermal annealing and their practical application. J. Mater. Sci.-Mater. El. 2021, 32, 16694–16705. [Google Scholar] [CrossRef]
- Zheng, J.; He, C.; Li, X.; Wang, K.; Wang, T.; Zhang, R.; Tang, B.; Rui, Y. CoS2-MnS@Carbon nanoparticles derived from metaleorganic framework as a promising anode for lithium-ion batteries. J. Alloy. Compd. 2021, 854, 157315. [Google Scholar] [CrossRef]
- Hu, D.; Zhu, C.; Yao, Y.; Liu, S.; Meng, X.; Yuan, H.; Chen, Z.; Jiang, X.; Li, Y.; Zhu, S. A facile ex situ strategy of α-MnS nanoparticles anchored on holey graphene as high-performance anode for lithium-ion batteries. Appl. Surf. Sci. 2020, 542, 148496. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Zhu, J.; Meng, T.; Ma, L.; Zhang, H.; Xu, M.; Jiang, J.; Li, C.M. One-dimensional integrated MnS@carbon nanoreactors hybrid: An alternative anode for full-cell Li-ion and Na-iIon bBatteries. ACS Appl. Mater. Interfaces 2018, 10, 27911–27919. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Y.; Kim, G.-T.; Diemant, T.; Behm, R.J.; Geiger, D.; Kaiser, U.; Varzi, A.; Passerini, S. Superior lithium storage capacity of α-MnS nanoparticles embedded in S-doped carbonaceous mesoporous frameworks. Adv. Energy Mater. 2019, 9, 1902077. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Cong, J.; Chen, Y.; Wang, Q.; Shi, M.; Liu, X.; Yang, H. MnS nanoparticles embedded in N,S co-doped carbon nanosheets for superior lithium ion storage. Appl. Surf. Sci. 2019, 508, 145239. [Google Scholar] [CrossRef]
- Ning, J.; Zhang, D.; Song, H.; Chen, X.; Zhou, J. Branched carbon-encapsulated MnS core/shell nanochains prepared via oriented attachment for lithium-ion storage. J. Mater. Chem. A 2016, 4, 12098–12105. [Google Scholar] [CrossRef]
- Gao, S.; Chen, G.; Dall’Agnese, Y.; Wei, Y.; Gao, Z.; Gao, Y. Flexible MnS-Carbon Fiber Hybrids for Lithium-Ion and Sodium-Ion Energy Storage. Chem.–A Eur. J. 2018, 24, 13535–13539. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, H.; Xiao, F.; Yao, T.; Liu, T.; Li, F.; Wang, J.; Han, X.; Cheng, Y.; Wang, H. Flower-like Mn/Co glycerolate-derived α-MnS/Co9S8/carbon heterostructures for high-performance lithium-ion batteries. ACS Appl. Energy Mater. 2020, 3, 10215–10223. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, C.; Yang, X.; Xiao, G.; Zou, B.; Yang, J.; Wang, C. Studies on intrinsic phase-dependent electrochemical properties of MnS nanocrystals as anodes for lithium-ion batteries. J. Power Sources 2017, 338, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Geng, H.; Su, H.; Lin, C.; Ma, Y.; Zhang, Y.; Chen, D.; Tan, H.; Rui, X.; Fang, Y.-X.; Li, C.C. Double-Layer N,S-Codoped Carbon Protection of MnS Nanoparticles Enabling Ultralong-Life and High-Rate Lithium Ion Storage. ACS Appl. Energy Mater. 2018, 1, 4867–4873. [Google Scholar] [CrossRef]
- Beltran-Huarac, J.; Resto, O.; Carpena-Nuñez, J.; Jadwisienczak, W.M.; Fonseca, L.F.; Weiner, B.R.; Morell, G. Single-Crystal γ-MnS Nanowires Conformally Coated with Carbon. ACS Appl. Mater. Interfaces 2014, 6, 1180–1186. [Google Scholar] [CrossRef]
- Zhou, X.-H.; Su, K.-M.; Kang, W.-M.; Cheng, B.-W.; Li, Z.-H. Nanosized α-MnS homogenously embedded in axial multichannel carbon nanofibers as freestanding electrodes for lithium-ion batteries. J. Mater. Sci. 2020, 55, 7403–7416. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Zdorovets, M. Synthesis, structural, strength and corrosion properties of thin films of the type CuX (X = Bi, Mg, Ni). J. Mater. Sci.-Mater. El. 2019, 30, 11819–11832. [Google Scholar] [CrossRef]
- Zubar, T.I.; Usovich, T.I.; Tishkevich, D.I.; Kanafyev, O.D.; Fedkin, V.A.; Kotelnikova, A.N.; Panasyuk, M.I.; Kurochka, A.S.; Nuriev, A.V.; Idris, A.M.; et al. Features of Galvanostatic Electrodeposition of NiFe Films with Composition Gradient: Influence of Substrate Characteristics. Nanomaterials 2022, 12, 2926. [Google Scholar] [CrossRef]
- Xu, X.; Ji, S.; Gu, M.; Liu, J. In Situ Synthesis of MnS Hollow Microspheres on Reduced Graphene Oxide Sheets as High-Capacity and Long-Life Anodes for Li- and Na-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 20957–20964. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Zdorovets, M. Effect of doping of Ce4+/3+ on optical, strength and shielding properties of (0.5-x)TeO2-0.25MoO-0.25Bi2O3-xCeO2 glasses. Mater. Chem. Phys. 2021, 263, 124444. [Google Scholar] [CrossRef]
- Almessiere, M.; Algarou, N.; Slimani, Y.; Sadaqat, A.; Baykal, A.; Manikandan, A.; Trukhanov, S.; Ercan, I. Investigation of exchange coupling and microwave properties of hard/soft (SrNi0.02Zr0.01Fe11.96O19)/(CoFe2O4)x nanocomposites. Mater. Today Nano 2022, 18, 100186. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, H.; Zhang, T.; Ma, L.; Ye, P.; Zhong, Y.; Hu, Y. Designed preparation of CoS/Co/MoC nanoparticles incorporated in N and S dual-doped porous carbon nanofibers for high-performance Zn-air batteries. Chin. Chem. Lett. 2020, 32, 2243–2248. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, F.; Li, S.; Lin, Z.; Huang, J. A cellulose substance derived nanofibrous CoS–nanoparticle/carbon composite as a high-performance anodic material for lithium-ion batteries. New J. Chem. 2020, 44, 1846–1857. [Google Scholar] [CrossRef]
- Gu, Y.; Xu, Y.; Wang, Y. Graphene-wrapped CoS nanoparticles for high-capacity lithium-ion storage. ACS Appl. Mater. Interfaces 2013, 5, 801–806. [Google Scholar] [CrossRef]
- Wang, H.; Ma, J.; Liu, S.; Nie, L.; Chai, Y.; Yang, X.; Yuan, R. CoS/CNTs hybrid structure for improved performance lithium ion battery. J. Alloy. Compd. 2016, 676, 551–556. [Google Scholar] [CrossRef]
- Tan, Y.; Liang, M.; Lou, P.; Cui, Z.; Guo, X.; Sun, W.; Yu, X. In situ fabrication of CoS and NiS nanomaterials anchored on reduced graphene oxide for reversible lithium storage. ACS Appl. Mater. Interfaces 2016, 8, 14488–14493. [Google Scholar] [CrossRef]
- Jin, P.; Zhang, X.; Wang, J. Mesoporous γ-MnS nanospheres as anode materials for Li ion batteries. Mater. Lett. 2017, 188, 13–16. [Google Scholar] [CrossRef]
- Camacho, R.A.P.; Wu, A.-M.; Jin, X.-Z.; Dong, X.-F.; Li, X.-N.; Huang, H. Effective carbon constraint of MnS nanoparticles as high-performance anode of lithium-ion batteries. J. Power Sources 2019, 437, 226931. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, J.; Huang, X.; Ding, J. Γ-MnS/reduced graphene oxide nanocomposites with great lithium storage capacity. Solid State Ionics 2015, 278, 138–143. [Google Scholar] [CrossRef]
- Yu, L.; Yang, J.; Lou, X. Formation of CoS2 nanobubble hollow prisms for highly reversible lithium storage. Angew. Chem. Int. Edit. 2016, 55, 13422–13426. [Google Scholar] [CrossRef]
- He, J.; Chen, Y.; Li, P.; Fu, F.; Wang, Z.; Zhang, W. Self-assembled CoS2 nanoparticles wrapped by CoS2-quantum-dots-anchored graphene nanosheets as superior-capability anode for lithium-ion batteries. Electrochim. Acta 2015, 182, 424–429. [Google Scholar] [CrossRef]









| Materials | Current Density (A g−1) | Specific Capacity (mAh g−1) | Cycling Performance | Ref. |
|---|---|---|---|---|
| CoS/Graphene | 0.06 | 749 | 40 | [39] |
| CoS/CNTs | 0.3 | 465 | 100 | [40] |
| CoS NFs-rGO | 0.1 | 939 | 100 | [41] |
| MnS/RGO | 1 | 640 | 400 | [34] |
| α-MnS | 0.1 | 870 | 200 | [21] |
| CoS2-MnS@rGO | 0.1 | 1324 | 100 | [20] |
| γ-MnS | 0.2 | 350 | 300 | [42] |
| MnS@C | 1 | 578 | 100 | [43] |
| γ-MnS/rGO | 0.2 | 600 | 100 | [44] |
| MnS@C-800 | 0.5 | 200 | 100 | [25] |
| CoS2 | 1 | 737 | 200 | [45] |
| CoS2 NP@G-CoS2 QD | 1 | 831 | 300 | [46] |
| MnS@CoS/C@C | 1 | 1001 | 100 | This work |
| 1 | 674 | 300 | ||
| 5 | 481 | 300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, P.; Yuan, Y.; Zhang, D.; Yang, Q.; Guo, S.; Cheng, J. Nanocapsule of MnS Nanopolyhedron Core@CoS Nanoparticle/Carbon Shell@Pure Carbon Shell as Anode Material for High-Performance Lithium Storage. Molecules 2023, 28, 898. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28020898
Yang P, Yuan Y, Zhang D, Yang Q, Guo S, Cheng J. Nanocapsule of MnS Nanopolyhedron Core@CoS Nanoparticle/Carbon Shell@Pure Carbon Shell as Anode Material for High-Performance Lithium Storage. Molecules. 2023; 28(2):898. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28020898
Chicago/Turabian StyleYang, Peng, Yongfeng Yuan, Dong Zhang, Qiuhe Yang, Shaoyi Guo, and Jipeng Cheng. 2023. "Nanocapsule of MnS Nanopolyhedron Core@CoS Nanoparticle/Carbon Shell@Pure Carbon Shell as Anode Material for High-Performance Lithium Storage" Molecules 28, no. 2: 898. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28020898