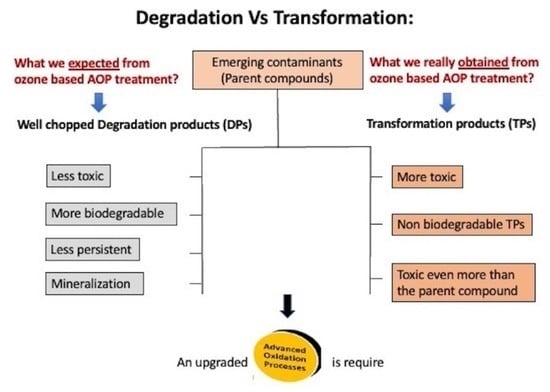
Pharmaceutical Transformation Products Formed by Ozonation—Does Degradation Occur?
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ozone and Ozone/H2O2
2.2. TPs of the PCs
2.3. Summary
3. Materials and Methods
3.1. Standards and Reagents
3.2. Laboratory Scale Ozone Experiments
3.3. Analytical Measurements
3.4. Transformation Products’ Identification
3.5. Toxicity Assessment Using the Ecological Structure–Activity Relationships Program (Ecosar 2.0)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Luo, Y.L.; Guo, W.S.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Ahmad, N.; Ali, J.; Hassan, A.A.; Sher, F.; Rizwan, K.; Iqbal, H.M.N.; Bilal, M. Nano and micro architectured cues as smart materials to mitigate recalcitrant pharmaceutical pollutants from wastewater. Chemosphere 2021, 274, 129785. [Google Scholar] [CrossRef]
- Carballa, M.; Omil, F.; Lema, J. Removal of cosmetic ingredients and pharmaceuticals in sewage primary treatment. Water Res. 2005, 39, 4790–4796. [Google Scholar] [CrossRef]
- Martini, J.; Orge, C.A.; Faria, J.L.; Pereira, M.F.R.; Soares, O.S.G. Sulfamethoxazole degradation by combination of advanced oxidation processes. J. Environ. Chem. Eng. 2018, 6, 4054–4060. [Google Scholar] [CrossRef]
- Craun, G.F.; Calderon, R.L. Workshop summary: Estimating waterborne disease risks in the United States. J. Water Heal. 2006, 4, 241–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, K.M.S.; Spiliotopoulou, A.; Chhetri, R.K.; Escolà Casas, M.; Bester, K.; Andersen, H.R. Ozonation for source treatment of pharmaceuticals in hospital wastewater—Ozone lifetime and required ozone dose. Chem. Eng. J. 2016, 290, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.; Yu, S.; Chen, W. Occurrence, removal and risk assessment of pharmaceutical and personal care products (PPCPs) in an advanced drinking water treatment plant (ADWTP) around Taihu Lake in China. Chemosphere 2016, 152, 1–9. [Google Scholar] [CrossRef]
- Paucar, N.E.; Kim, I.; Tanaka, H.; Sato, C. Ozone treatment process for the removal of pharmaceuticals and personal care products in wastewater. Ozone: Sci. Eng. 2018, 41, 3–16. [Google Scholar] [CrossRef]
- Mishra, N.; Reddy, R.; Kuila, A.; Rani, A.; Nawaz, A.; Pichiah, S. A Review on Advanced Oxidation Processes for Effective Water Treatment. Curr. World Environ. J. 2017, 12, 469–489. [Google Scholar] [CrossRef]
- Gunten, U.V. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef] [PubMed]
- Von Gunten, U. Oxidation processes in water treatment: Are we on track? Environ. Sci. Technol. 2018, 52, 5062–5075. [Google Scholar] [CrossRef] [PubMed]
- Ramseier, M.K.; Von Gunten, U. Mechanisms of Phenol Ozonation—Kinetics of Formation of Primary and Secondary Reaction Products. Ozone: Sci. Eng. 2009, 31, 201–215. [Google Scholar] [CrossRef]
- Lajeunesse, A.; Blais, M.; Barbeau, B.; Sauvé, S.; Gagnon, C. Ozone oxidation of antidepressants in wastewater—Treatment evaluation and characterization of new by-products by LC-QToFMS. Chem. Central J. 2013, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Mvula, E.; von Sonntag, C. Ozonolysis of phenols in aqueous solution. Org. Biomol. Chem. 2003, 1, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, M.; Gupta, M.; Agrahari, S.K.; Detwal, P. Removal of Refractory Organic Compounds from Wastewater by Various Advanced Oxidation Process—A Review. Curr. Environ. Eng. 2019, 6, 8–16. [Google Scholar] [CrossRef]
- Duirk, S.E.; Lindell, C.; Cornelison, C.C.; Kormos, J.; Ternes, T.A.; Attene-Ramos, M.; Osiol, J.; Wagner, E.D.; Plewa, M.J.; Richardson, S.D. Formation of Toxic Iodinated Disinfection By-Products from Compounds Used in Medical Imaging. Environ. Sci. Technol. 2011, 45, 6845–6854. [Google Scholar] [CrossRef]
- Hu, C.-Y.; Du, Y.-F.; Lin, Y.-L.; Hua, S.-J.; Hou, Y.-Z.; Deng, Y.-G.; Zhang, J.-C.; Ren, S.-C.; Dong, C.-D.; Chen, C.-W.; et al. Kinetics of iohexol degradation by ozonation and formation of DBPs during post-chlorination. J. Water Process. Eng. 2020, 35, 101200. [Google Scholar] [CrossRef]
- Akao, P.K.; Mamane, H.; Kaplan, A.; Gozlan, I.; Yehoshua, Y.; Kinel-Tahan, Y.; Avisar, D. Iohexol removal and degradation-product formation via biodegradation by the microalga Chlorella vulgaris. Algal Res. 2020, 51. [Google Scholar] [CrossRef]
- Giannakis, S.; Jovic, M.; Gasilova, N.; Gelabert, M.P.; Schindelholz, S.; Furbringer, J.-M.; Girault, H.; Pulgarin, C. Iohexol degradation in wastewater and urine by UV-based Advanced Oxidation Processes (AOPs): Process modeling and by-products identification. J. Environ. Manag. 2017, 195, 174–185. [Google Scholar] [CrossRef] [Green Version]
- Jeong, C.H.; Machek, E.J.; Shakeri, M.; Duirk, S.E.; Ternes, T.A.; Richardson, S.D.; Wagner, E.D.; Plewa, M.J. The impact of iodinated X-ray contrast agents on formation and toxicity of disinfection by-products in drinking water. J. Environ. Sci. 2017, 58, 173–182. [Google Scholar] [CrossRef]
- Huber, M.M.; Canonica, S.; Park, G.-Y.; von Gunten, U. Oxidation of Pharmaceuticals during Ozonation and Advanced Oxidation Processes. Environ. Sci. Technol. 2003, 37, 1016–1024. [Google Scholar] [CrossRef]
- López-Serna, R.; Jurado, A.; Vázquez-Suñé, E.; Carrera, J.; Petrović, M.; Barceló, D. Occurrence of 95 pharmaceuticals and transformation products in urban groundwaters underlying the metropolis of Barcelona, Spain. Environ. Pollut. 2013, 174, 305–315. [Google Scholar] [CrossRef]
- Radjenović, J.; Petrović, M.; Barceló, D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res. 2009, 43, 831–841. [Google Scholar] [CrossRef]
- Rocco, L.; Peluso, C.; Cesaroni, F.; Morra, N.; Stingo, V. Genomic Damage in Human Sperm Cells Exposed In Vitro to Environmental Pollutants. J. Environ. Anal. Toxicol. 2012, 2. [Google Scholar] [CrossRef]
- Sui, Q.; Gebhardt, W.; Schröder, H.F.; Zhao, W.; Lu, S.; Yu, G. Identification of New Oxidation Products of Bezafibrate for Better Understanding of Its Toxicity Evolution and Oxidation Mechanisms during Ozonation. Environ. Sci. Technol. 2017, 51, 2262–2270. [Google Scholar] [CrossRef]
- Dantas, R.F.; Canterino, M.; Marotta, R.; Sans, C.; Esplugas, S.; Andreozzi, R. Bezafibrate removal by means of ozonation: Primary intermediates, kinetics, and toxicity assessment. Water Res. 2007, 41, 2525–2532. [Google Scholar] [CrossRef]
- Gonçalves, A.; Órfão, J.; Pereira, M.F. Ozonation of bezafibrate promoted by carbon materials. Appl. Catal. B: Environ. 2013, 140-141, 82–91. [Google Scholar] [CrossRef]
- Rivas, F.J.; Solís, R.R.; Beltrán, F.J.; Gimeno, O. Sunlight driven photolytic ozonation as an advanced oxidation process in the oxidation of bezafibrate, cotinine and iopamidol. Water Res. 2018, 151, 226–242. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Liu, Y.; Zhang, L.; Feng, L. Modelling study on the effects of chloride on the degradation of bezafibrate and carbamazepine in sulfate radical-based advanced oxidation processes: Conversion of reactive radicals. Chem. Eng. J. 2018, 358, 1332–1341. [Google Scholar] [CrossRef]
- Chen, X.; Yao, J.; Dong, H.; Hong, M.; Gao, N.; Zhang, Z.; Jiang, W. Enhanced bezafibrate degradation and power generation via the simultaneous PMS activation in visible light photocatalytic fuel cell. Water Res. 2021, 207, 117800. [Google Scholar] [CrossRef]
- Ding, T.; Wang, S.; Yang, B.; Li, J. Biological removal of pharmaceuticals by Navicula sp. and biotransformation of bezafibrate. Chemosphere 2020, 240, 124949. [Google Scholar] [CrossRef]
- Ellenberger, J.; Mohn, G. Mutagenic activity of cyclophosphamide, ifosfamide, and trofosfamide in different genes of Escherichia coli and Salmonella typhimurium after biotransformation through extracts of rodent liver. Arch. Toxicol. 1975, 33, 225–240. [Google Scholar] [CrossRef]
- Witt, K.L.; Bishop, J.B. Mutagenicity of anticancer drugs in mammalian germ cells. Mutat. Res. Mol. Mech. Mutagen. 1996, 355, 209–234. [Google Scholar] [CrossRef]
- Osawa, R.A.; Barrocas, B.T.; Monteiro, O.C.; Oliveira, M.C.; Florêncio, M.H. Photocatalytic degradation of cyclophosphamide and ifosfamide: Effects of wastewater matrix, transformation products and in silico toxicity prediction. Sci. Total. Environ. 2019, 692, 503–510. [Google Scholar] [CrossRef]
- Blaney, L.; Lawler, D.F.; Katz, L.E. Transformation kinetics of cyclophosphamide and ifosfamide by ozone and hydroxyl radicals using continuous oxidant addition reactors. J. Hazard. Mater. 2018, 364, 752–761. [Google Scholar] [CrossRef]
- Abou-Eisha, A.; Marcos, R.; Creus, A. Genotoxicity studies on the antimicrobial drug sulfamethoxazole in cultured human lymphocytes. Mutat. Res. Toxicol. Environ. Mutagen. 2004, 564, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Lofton, A.L.; Klein-Schwartz, W. Evaluation of Lamotrigine Toxicity Reported to Poison Centers. Ann. Pharmacother. 2004, 38, 1811–1815. [Google Scholar] [CrossRef]
- Keen, O.S.; Ferrer, I.; Thurman, E.M.; Linden, K.G. Degradation pathways of lamotrigine under advanced treatment by direct UV photolysis, hydroxyl radicals, and ozone. Chemosphere 2014, 117, 316–323. [Google Scholar] [CrossRef]
- Goldstein, M.; Malchi, T.; Shenker, M.; Chefetz, B. Pharmacokinetics in Plants: Carbamazepine and Its Interactions with Lamotrigine. Environ. Sci. Technol. 2018, 52, 6957–6964. [Google Scholar] [CrossRef]
- Diehle, M.; Gebhardt, W.; Pinnekamp, J.; Schäffer, A.; Linnemann, V. Ozonation of valsartan: Structural elucidation and environmental properties of transformation products. Chemosphere 2018, 216, 437–448. [Google Scholar] [CrossRef]
- Dodd, M.C.; Buffle, M.-O.; von Gunten, U. Oxidation of Antibacterial Molecules by Aqueous Ozone: Moiety-Specific Reaction Kinetics and Application to Ozone-Based Wastewater Treatment. Environ. Sci. Technol. 2006, 40, 1969–1977. [Google Scholar] [CrossRef]
- Mezyk, S.P.; Neubauer, T.J.; Cooper, W.J.; Peller, J.R. Free-Radical-Induced Oxidative and Reductive Degradation of Sulfa Drugs in Water: Absolute Kinetics and Efficiencies of Hydroxyl Radical and Hydrated Electron Reactions. J. Phys. Chem. A 2007, 111, 9019–9024. [Google Scholar] [CrossRef]
- Ramos, M.D.M.G.; Mezcua, M.; Agüera, A.; Fernández-Alba, A.R.; Gonzalo, S.; Rodríguez, A.; Rosal, R. Chemical and toxicological evolution of the antibiotic sulfamethoxazole under ozone treatment in water solution. J. Hazard. Mater. 2011, 192, 18–25. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, Z.; Xu, Y.; Tian, J.; Qi, H.; Lin, W.; Cui, F. Oxidation of sulfamethoxazole (SMX) by chlorine, ozone and permanganate—A comparative study. J. Hazard. Mater. 2014, 274, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gong, Q.; Ali, J.; Shen, M.; Cai, J.; Zhou, X.; Liao, Z.; Wang, S.; Chen, Z. pH-dependent transformation products and residual toxicity evaluation of sulfamethoxazole degradation through non-radical oxygen species involved process. Chem. Eng. J. 2020, 390, 124512. [Google Scholar] [CrossRef]
- Gulde, R.; Rutsch, M.; Clerc, B.; Schollée, J.E.; von Gunten, U.; McArdell, C.S. Formation of Transformation Products during Ozonation of Secondary Wastewater Effluent and Their Fate in Post-Treatment: From Laboratory- to Full-Scale. Water Res. 2021, 200, 117200. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.Y.; Lim, F.Y.; Loh, W.H.; Ong, S.L.; Hu, J. Emerging Contaminants: An Overview of Recent Trends for Their Treatment and Management Using Light-Driven Processes. Water 2021, 13, 2340. [Google Scholar] [CrossRef]
- Baus, C.; Sacher, F.; Brauch, D. Oxidation of iodinated X-ray contrast media in water samples—results of laboratory scale experiments. In Proceedings of the 70th Annual Conference of the Water Chemical Society, Division of the German Chemical Society, Bad Saarow, Germany, May 2004. [Google Scholar]
- Lee, Y.; Kovalova, L.; McArdell, C.S.; von Gunten, U. Prediction of micropollutant elimination during ozonation of a hospital wastewater effluent. Water Res. 2014, 64, 134–148. [Google Scholar] [CrossRef] [Green Version]
- Lester, Y.; Avisar, D.; Gozlan, I.; Mamane, H. Removal of pharmaceuticals using combination of UV/H2O2/O3 advanced oxidation process. Water Sci. Technol. 2011, 64, 2230–2238. [Google Scholar] [CrossRef] [Green Version]
- Bader, H.; Hoigné, J. Determination of ozone in water by the indigo method. Water Res. 1981, 15, 449–456. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Liu, C.; Fang, G.; Chu, L.; Gu, C.; Gao, J. Persistent Free Radicals from Low-Molecular-Weight Organic Compounds Enhance Cross-Coupling Reactions and Toxicity of Anthracene on Amorphous Silica Surfaces under Light. Environ. Sci. Technol. 2021, 55, 3716–3726. [Google Scholar] [CrossRef] [PubMed]







| IHX | CYP | BZF | LMG | VAL | SMX | |
|---|---|---|---|---|---|---|
| TOC [ppm] Ozonation at t = 0 min | 5.31 | 4.51 | 4.43 | 4.66 | 6.11 | 5.40 |
| TOC [ppm] Ozonation at t = 60 min | 5.26 | 4.59 | 3.16 | 4.65 | 5.93 | 4.66 |
| Abs [mAU] at 254 nm for O3 t = 0 min | 34 | 1 | 10 | 21 | 33 | 50 |
| Abs [mAU] at 254 nm for O3 t = 20 min | 60 | 29 | 33 | 41 | 37 | 36 |
| No | Rt [min] | [M+H]+ [Da] | Formula | Ref. | Suggested Occurrence | ||
|---|---|---|---|---|---|---|---|
| Obtained Mass | Calculated Mass | Deviation (ppm) | |||||
| Iohexol (IHX) | 4.91, 6.01 | 821.8934 | 821.8876 | −7.1 | C19H26I3N3O9 | [18,19,20] | |
| ID851 | 4.26, 4.8 | 851.8254 | 851.8253 | 0.0 | C18H20I3N3O12 | Oxidation of three OH groups to obtain three carboxylic acid | |
| ID621 | 4.45 | 621.9617 | 621.9541 | −12.1 | C16H21I2N3O7 | [18] | Elimination of dihydroxypropyl group Elimination of iodine atom |
| ID819 | 5.92, 6.34 | 819.8809 | 819.8719 | −11.0 | C19H24I3N3O9 | [18] | Oxidation of OH group to aldehyde |
| ID747 | 6.35 | 747.8533 | 747.8508 | −3.3 | C16H20I3N3O7 | [18] | Elimination of dihydroxypropyl group |
| ID673 | 7.32 | 673.8154 | 673.8140 | −2.1 | C13H14I3N3O5 | [18] | Elimination of two dihydroxypropyl groups |
| No | Rt [min] | [M+H]+ [Da] | Formula | Ref. | Suggested Occurrence | ||
|---|---|---|---|---|---|---|---|
| Obtained Mass | Calculated Mass | Deviation (ppm) | |||||
| Bezafibrate (BZF) | 15.37 | 362.1151 | 362.1154 | 0.7 | C19H20ClNO4 | [26,27,28] | |
| ID292 | 12.89 | 292.0708 | 292.0735 | 9.2 | C15H14ClNO3 | [25,29,30] | Elimination of 2-methylpropanoic acid group and hydroxylation of aliphatic group |
| ID368 | 14.49 | 368.0883 | 368.0895 | 3.4 | C17H18ClNO6 | [31] | Two steps:
|
| ID290 | 14.82 | 290.0579 | 290.0578 | −0.2 | C15H12ClNO3 | [25] | Elimination of H2O from ID292 to obtain double bond Hydroxylation of aromatic ring |
| ID394 | 14.91 | 394.1005 | 394.1052 | 11.9 | C19H20ClNO6 | [25,29,30] | Hydroxylation of chloroaromatic ring |
| ID318 | 15.59 | 318.0895 | 318.0891 | −1.3 | C17H16ClNO3 | ||
| No | Rt [min] | [M+H]+ [Da] | Formula | Ref. | Suggested Occurrence | ||
|---|---|---|---|---|---|---|---|
| Obtained Mass | Calculated Mass | Deviation (ppm) | |||||
| Cyclophosphamide (CYP) | 12.15 12.61 | 261.0330 | 261.0321 | −3.4 | C7H15Cl2N2O2P | [34,35] | |
| ID199 | 7.27 | 199.0386 | 199.0398 | 5.9 | C5H12ClN2O2P | [34,35] | Elimination of chloroethyl group |
| ID275 | 10.53 | 275.0096 | 275.0114 | 6.4 | C7H13Cl2N2O3P | [34,35] | Oxidation of the oxazaphosphinan group (possibly obtained from product ID277) |
| ID277 | 10.53 | 277.0212 | 277.0270 | 21.0 | C7H15Cl2N2O3P | [34,36] | Hydroxylation of the oxazaphosphinan group |
| ID259 | 11.72 | 259.0150 | 259.0164 | 5.6 | C7H13Cl2N2O2P | [35] | Elimination of H2O from product ID277 |
| No | Rt [min] | [M+H]+ [Da] | Formula | Ref. | Suggested Occurrence | ||
|---|---|---|---|---|---|---|---|
| Obtained mass | Calculated mass | Deviation (ppm) | |||||
| Lamotrigine (LMG) | 10.12 | 256.0144 | 256.0151 | 2.9 | C9H7Cl2N5 | [38] | |
| ID270 | 3.12, 3.86 | 270.0369 | 270.0388 | 7.2 | C9H8ClN5O3 | Oxidation of amine group to nitro group | |
| ID306 | 3.58, 3.77, 4.13, 4.32 | 306.0133 | 306.0155 | 7.3 | C9H9Cl2N5O3 | Two steps:
| |
| ID238 | 5.09, 7.12 | 238.0487 | 238.0490 | 1.3 | C9H8ClN5O | [38] | Exchange of chlorine atom with hydroxyl group—two possible substitution options |
| ID272 | 8.92, 9.85 | 272.0112 | 272.0100 | −4.3 | C9H7Cl2N5O | [38] | Hydroxylation of aromatic ring |
| No | Rt [min] | [M+H]+ [Da] | Formula | Ref. | Suggested Occurrence | ||
|---|---|---|---|---|---|---|---|
| Obtained Mass | Calculated Mass | Deviation (ppm) | |||||
| Valsartan (Val) | 15.93 | 436.2324 | 436.2343 | 4.4 | C24H29N5O3 | [40] | |
| ID366 | 13.81 | 366.15647 | 366.1561 | −1.1 | C19H19N5O3 | [40] | Oxidation of methylene group and elimination of the pentanoyl group |
| ID452 | 14.54 | 452.2324 | 452.2292 | −7.0 | C24H29N5O4 | [40] | Hydroxylation of aromatic ring (more than one option of each aromatic ring) |
| ID450 | 14.91 | 450.2141 | 450.2139 | −1.2 | C24H27N5O4 | [40] | Oxidation of methyl group to aldehyde group |
| No | Rt [min] | [M+H]+ [Da] | Formula | Ref. | Suggested Occurrence | ||
|---|---|---|---|---|---|---|---|
| Obtained Mass | Calculated Mass | Deviation (ppm) | |||||
| Sulfamethoxazole (SMX) | 11.41 | 254.0586 | 254.0594 | 3.1 | C10H11N3O3S | [4,44] | |
| ID284 | 8.83, 9.98 | 284.0327 | 284.0336 | 3.1 | C10H9N3O5S | [44,45] | Oxidation of amine group to nitro group |
| ID270 | 8.78, 10.86 | 270.0549 | 270.0543 | −2.2 | C10H11N3O4S | [44,45] | Hydroxylation of aromatic ring |
| ID99 | 4.26 | 99.0545 | 99.0553 | 8.0 | C4H7N2O | [44,45] | Cleavage of N-S bond of the sulfonamide group |
| Name | Class | pKa * | Rate Constants | Ref. | |
|---|---|---|---|---|---|
| kO3 [M−1 s−1] | k∙OH [109 M−1 s−1] | ||||
| Iohexol (IHX) | Contrast media | 11.3 | 1.4 | 3.3 | [21,48] |
| Bezafibrate (BZF) | Lipid regulator | 3.3 (acid) | 590 | 7.4 | [21] |
| Lamotrigine (LMG) | Anticonvulsant | 5.4 (base) | 4 | 2.1 | [38] |
| Valsartan (VAL) | Blood pressure | 3.6, 4.2 (acid) | 38 | 10 | [49] |
| Cyclophosphamide (CYP) | Anticancer | 2.84 | 2.8 | 1.3 | [50] |
| Sulfamethoxazole (SMX) | Antibiotic | 5.8 (acid) | 4.7 × 104 5.7 × 105 | 5.5, 8.5 | [21,41,42] |
| Compound | Iohexol (IHX) | Bezafibrate (BZF) | Lamotrigine (LMG) | Valsartan (VAL) | Cyclophosphamide (CYP) | Sulfamethoxazole (SMX) |
|---|---|---|---|---|---|---|
| RT (min) | 4.4, 5.2 | 15.02 | 9.7 | 15.6 | 12.01 | 10.87 |
| [MH]+ | 821.893 | 362.115 | 256.014 | 436.232 | 261.033 | 254.059 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zilberman, A.; Gozlan, I.; Avisar, D. Pharmaceutical Transformation Products Formed by Ozonation—Does Degradation Occur? Molecules 2023, 28, 1227. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28031227
Zilberman A, Gozlan I, Avisar D. Pharmaceutical Transformation Products Formed by Ozonation—Does Degradation Occur? Molecules. 2023; 28(3):1227. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28031227
Chicago/Turabian StyleZilberman, Adi, Igal Gozlan, and Dror Avisar. 2023. "Pharmaceutical Transformation Products Formed by Ozonation—Does Degradation Occur?" Molecules 28, no. 3: 1227. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28031227