Green-Synthesization of Silver Nanoparticles Using Endophytic Bacteria Isolated from Garlic and Its Antifungal Activity against Wheat Fusarium Head Blight Pathogen Fusarium graminearum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms Used in This Study
2.2. Biosynthesis of AgNPs
2.3. Antifungal Activity of the Biosynthesized AgNPs
2.3.1. Inhibition of AgNPs on Mycelium Growth
2.3.2. Effect of AgNPs on Spore Germination and Length of Germ Tubes
2.3.3. Effect of AgNPs on Deoxynivalenol Production
2.4. Antifungal Mechanism of the Biosynthesized AgNPs
2.4.1. Effect of AgNPs on the Hyphal Morphology
2.4.2. Effect of AgNPs on the Leakage of DNA and Proteins
2.5. Characterization of the Biosynthesized AgNPs
2.6. Statistical Analysis
3. Results and Discussion
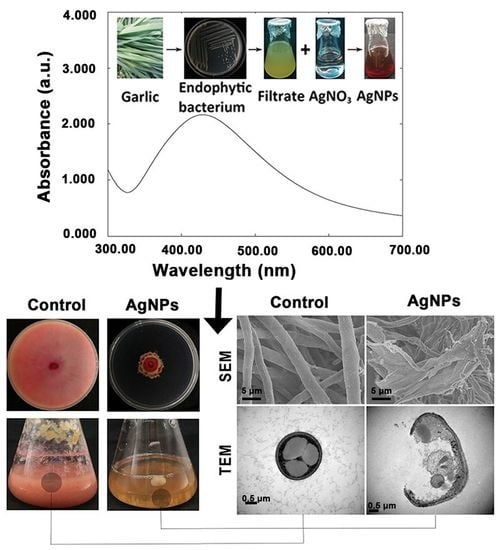
3.1. Biosynthesis and Confirmation of AgNPs
3.2. Antifungal Activity and Mechanism
3.2.1. Inhibition of Mycelium Growth
3.2.2. Damage of Cell Walls by Morphological Observation
3.2.3. Damage of Cell Walls by Determination of Leakage DNA and Proteins
3.2.4. Inhibition of Spore Germination and Germ Tube Growth
3.2.5. Effect of AgNPs on DON Production
3.3. Characterization of the Biosynthesized AgNPs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dubin, H.J.; Gilchrist, L.; Reeves, J.; McNab, A. Fusarium Head Scab: Global Status and Future Prospects; E-Publishing, Inc.: El Batan, Mexico, 1996; pp. 13–17. [Google Scholar]
- Goswami, R.S.; Kistler, H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant. Pathol. 2004, 5, 515–525. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, S.; Li, X.; Xu, J.-R.; Schultzhaus, Z.; Jin, Q.A. Gin4-Like Protein Kinase GIL1 Involvement in Hyphal Growth, Asexual Development, and Pathogenesis in Fusarium graminearum. Int. J. Mol. Sci. 2017, 18, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Kistler, H.C.; Ma, Z. Fusarium graminearum Trichothecene Mycotoxins: Biosynthesis, Regulation, and Management. Annu. Rev. Phytopathol. 2019, 57, 15–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hongxiang, M.; Yao, J.; Zhou, M.; Zhang, X.; Ren, L.; Yu, G. Molecular breeding for wheat fusarium head blight resistance in Cihina. Cereal Res. Commun. 2008, 36, 203–212. [Google Scholar] [CrossRef]
- Wu, D.; Wan, J.; Lu, J.; Wang, X.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Chitosan coatings on lecithin stabilized emulsions inhibit mycotoxin production by Fusarium pathogens. Food Control. 2018, 92, 276–285. [Google Scholar] [CrossRef]
- Wu, D.; Lu, J.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Effect of chitosan coatings on physical stability, antifungal and mycotoxin inhibitory activities of lecithin stabilized cinnamon oil-in-water emulsions. Lebensm-Wiss Technol. 2019, 106, 98–104. [Google Scholar] [CrossRef]
- Lakshmeesha, T.R.; Kalagatur, N.K.; Mudili, V.; Mohan, C.D.; Rangappa, S.; Prasad, B.D.; Hashem, A.; Alqarawi, A.A.; Malik, J.A.; Abd_Allah, E.F.; et al. Biofabrication of Zinc Oxide Nanoparticles With Syzygium aromaticum Flower Buds Extract and Finding Its Novel Application in Controlling the Growth and Mycotoxins of Fusarium graminearum. Front. Microbiol. 2019, 10, 1244. [Google Scholar] [CrossRef] [Green Version]
- Palazzini, J.M.; Dunlap, C.A.; Bowman, M.J.; Chulze, S.N. Bacillus velezensis RC 218 as a biocontrol agent to reduce Fusarium head blight and deoxynivalenol accumulation: Genome sequencing and secondary metabolite cluster profiles. Microbiol. Res. 2016, 192, 30–36. [Google Scholar] [CrossRef]
- Chen, L.; Heng, J.; Qin, S.; Bian, K.A. comprehensive understanding of the biocontrol potential of Bacillus velezensis LM2303 against Fusarium head blight. PloS ONE 2018, 13, e0198560. [Google Scholar] [CrossRef] [Green Version]
- Pariona, N.; Mtz-Enriquez, A.I.; Sánchez-Rangel, D.; Carrión, G.; Paraguay-Delgado, F.; Rosas-Saito, G. Green-synthesized copper nanoparticles as a potential antifungal against plant pathogens. RSC Adv. 2019, 9, 18835–18843. [Google Scholar] [CrossRef] [Green Version]
- Maluin, F.N.; Hussein, M.Z.; Yusof, N.A.; Fakurazi, S.; Idris, A.S.; Zainol Hilmi, N.H. Preparation of Chitosan-Hexaconazole Nanoparticles as Fungicide Nanodelivery System for Combating Ganoderma Disease in Oil Palm. Molecules 2019, 24, 2498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdallah, Y.; Ogunyemi, S.O.; Abdelazez, A.; Zhang, M.; Hong, X.; Ibrahim, E. The Green Synthesis of MgO Nano-Flowers Using Rosmarinus officinalis L. (Rosemary) and the Antibacterial Activities against Xanthomonas oryzae pv. oryzae. Biomed. Res. Int. 2019, 2019, 5620989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Use of copper, silver and zinc nanoparticles against foliar and soil-borne plant pathogens. Sci. Total Environ. 2019, 670, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.M.; El-Ghany, M.N.A.; Rodríguez-Couto, S. Antifungal and anti-mycotoxin efficacy of biogenic silver nanoparticles produced by Fusarium chlamydosporum and Penicillium chrysogenum at non-cytotoxic doses. Chemosphere 2019, 218, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Fouad, H.; Hongjie, L.; Hosni, D.; Wei, J.; Abbas, G.; Ga’al, H. Controlling Aedes albopictus and Culex pipiens pallens using silver nanoparticles synthesized from aqueous extract of Cassia fistula fruit pulp and its mode of action. rtif. Cells Nanomed. Biotechnol. 2018, 46, 558–567. [Google Scholar] [CrossRef] [Green Version]
- Masum, M.M.I.; Siddiqa, M.M.; Ali, K.A.; Zhang, Y.; Abdallah, Y.; Ibrahim, E. Biogenic Synthesis of Silver Nanoparticles Using Phyllanthus emblica Fruit Extract and Its Inhibitory Action Against the Pathogen Acidovorax oryzae Strain RS-2 of Rice Bacterial Brown Stripe. Front Microbiol. 2019, 10, 820. [Google Scholar] [CrossRef]
- Ogunyemi, S.O.; Abdallah, Y.; Zhang, M.; Fouad, H.; Hong, X.; Ibrahim, E. Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. oryzae. Artif. Cells Nanomed. Biotechnol. 2019, 47, 341–352. [Google Scholar] [CrossRef] [Green Version]
- Hossain, A.; Hong, X.; Ibrahim, E.; Li, B.; Sun, G.; Meng, Y. Green Synthesis of Silver Nanoparticles with Culture Supernatant of a Bacterium Pseudomonas rhodesiae and Their Antibacterial Activity against Soft Rot Pathogen Dickeya dadantii. Molecules. Molecules 2019, 24, 2303. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Gao, Q.; Hamada, M.S.; Dawood, D.H.; Zheng, J.; Chen, Y. Potential of Pseudomonas chlororaphis subsp. aurantiaca strain Pcho10 as a biocontrol agent against Fusarium graminearum. Phytopathol. 2014, 104, 1289–1297. [Google Scholar]
- Fouad, H.; Hongjie, L.; Yanmei, D.; Baoting, Y.; El-Shakh, A.; Abbas, G. Synthesis and characterization of silver nanoparticles using Bacillus amyloliquefaciens and Bacillus subtilis to control filarial vector Culex pipiens pallens and its antimicrobial activity. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1369–1378. [Google Scholar] [CrossRef] [Green Version]
- Spence, C.; Alff, E.; Johnson, C.; Ramos, C.; Donofrio, N.; Sundaresan, V. Natural rice rhizospheric microbes suppress rice blast infections. BMC Plant Biol. 2014, 14, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lu, J.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Influence of nonionic and ionic surfactants on the antifungal and mycotoxin inhibitory efficacy of cinnamon oil nanoemulsions. Food Funct. 2019, 10, 2817–2827. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Duan, Y.; Bian, C.; Pan, X.; Yao, C.; Wang, J. Effects of validamycin in controlling Fusarium head blight caused by Fusarium graminearum: Inhibition of DON biosynthesis and induction of host resistance. Pestic. Biochem. Physiol. 2019, 153, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Zhou, H.; Zhou, W.; Hu, L.; Chen, J.; Shi, Z. The Fungicidal Activity of Thymol against Fusarium graminearum via Inducing Lipid Peroxidation and Disrupting Ergosterol Biosynthesis. Molecules 2016, 21, 770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sastry, M.; Mayya, K.; Bandyopadhyay, K. pH Dependent changes in the optical properties of carboxylic acid derivatized silver colloidal particles. Colloids Surface A 1997, 127, 221–228. [Google Scholar] [CrossRef]
- Kasithevar, M.; Saravanan, M.; Prakash, P.; Kumar, H.; Ovais, M.; Barabadi, H. Green synthesis of silver nanoparticles using Alysicarpus monilifer leaf extract and its antibacterial activity against MRSA and CoNS isolates in HIV patients. J. Interdiscip. Nanomed. 2017, 2, 131–141. [Google Scholar] [CrossRef]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef] [Green Version]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanopart. Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Kaur, P.; Thakur, R.; Duhan, J.S.; Chaudhury, A. Management of wilt disease of chickpea in vivo by silver nanoparticles biosynthesized by rhizospheric microflora of chickpea (Cicer arietinum). J. Chem. Technol. Biot. 2018, 93, 3233–3243. [Google Scholar] [CrossRef]
- Bernardo-Mazariegos, E.; Valdez-Salas, B.; González-Mendoza, D.; Abdelmoteleb, A.; Camacho, O.T.; Duran, C.C. Silver nanoparticles from Justicia spicigera and their antimicrobial potentialities in the biocontrol of foodborne bacteria and phytopathogenic fungi. Rev. Argent. Microbiol. 2019, 51, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, M.; Anand, R.; Datt, R.; Gupta, V.; Arya, S. Green Synthesis of Silver Nanoparticles Using Aqueous Extract of Rosa brunonii Lindl and Their Morphological, Biological and Photocatalytic Characterizations. J. Inorg. Organomet. P. 2019, 29, 1039–1047. [Google Scholar] [CrossRef]
- Elgorban, A.M.; El-Samawaty, A.E.-R.M.; Yassin, M.A.; Sayed, S.R.; Adil, S.F.; Elhindi, K.M. Antifungal silver nanoparticles: Synthesis, characterization and biological evaluation. Biotechnol. Biotec. Eq. 2016, 30, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Xu, C.; Zhao, M.; Zhao, C. One-pot synthesis of narrowly distributed silver nanoparticles using phenolic-hydroxyl modified chitosan and their antimicrobial activity. RSC Advances 2014, 4, 47021–47030. [Google Scholar] [CrossRef]
- Yoshimi, A.; Miyazawa, K.; Abe, K. Function and biosynthesis of cell wall α−1, 3-glucan in fungi. J. Fungi. 2017, 3, 63. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.-K.; Ma, Q.-H.; Li, S.-Y.; Zhang, D.-Q.; Cong, L.; Tian, Y.-L. The antifungal effect of silver nanoparticles on Trichosporon asahii. J. Microbiol. Immunol. 2016, 49, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Setlow, P. Spore germination. Curr. Opin. Microbiol. 2003, 6, 550–556. [Google Scholar] [CrossRef]
- Hanif, A.; Zhang, F.; Li, P.; Li, C.; Xu, Y.; Zubair, M. Fengycin Produced by Bacillus amyloliquefaciens FZB42 Inhibits Fusarium graminearum Growth and Mycotoxins Biosynthesis. Toxins 2019, 11, 295. [Google Scholar] [CrossRef] [Green Version]
- Rasaee, I.; Ghannadnia, M.; Baghshahi, S. Biosynthesis of silver nanoparticles using leaf extract of Satureja hortensis treated with NaCl and its antibacterial properties. Micropor. Mesopor. Mat. 2018, 264, 240–247. [Google Scholar] [CrossRef]
- Vanaja, M.; Gnanajobitha, G.; Paulkumar, K.; Rajeshkumar, S.; Malarkodi, C.; Annadurai, G. Phytosynthesis of silver nanoparticles by Cissus quadrangularis: Influence of physicochemical factors. J. Nanostruct. Chem. 2013, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Benelli, G.; Kadaikunnan, S.; Alharbi, N.S.; Govindarajan, M. Biophysical characterization of Acacia caesia-fabricated silver nanoparticles: Effectiveness on mosquito vectors of public health relevance and impact on non-target aquatic biocontrol agents. Environ. Sci. Pollut. Res. Int. 2018, 25, 10228–10242. [Google Scholar] [CrossRef] [PubMed]
- Leopold, L.F.; Leopold, N.; Diehl, H.-A.; Socaciu, C. Quantification of carbohydrates in fruit juices using FTIR spectroscopy and multivariate analysis. J. Spectrosc. 2011, 26, 93–104. [Google Scholar] [CrossRef]
- Ghramh, H.A.; Al-Ghamdi, K.M.; Mahyoub, J.A.; Ibrahim, E.H. Chrysanthemum extract and extract prepared silver nanoparticles as biocides to control Aedes aegypti (L.), the vector of dengue fever. J. Asia-Pac. Entomol. 2018, 21, 205–210. [Google Scholar] [CrossRef]
- Kiran, G.S.; Sabu, A.; Selvin, J. Synthesis of silver nanoparticles by glycolipid biosurfactant produced from marine Brevibacterium casei MSA19. J. Biotechnol. 2010, 148, 221–225. [Google Scholar] [CrossRef]
- Velu, K.; Elumalai, D.; Hemalatha, P.; Janaki, A.; Babu, M.; Hemavathi, M. Evaluation of silver nanoparticles toxicity of Arachis hypogaea peel extracts and its larvicidal activity against malaria and dengue vectors. Environ. Sci. Pollut. Res. Int. 2015, 22, 17769–17779. [Google Scholar] [CrossRef]
- Banu, A.N.; Balasubramanian, C. Extracellular synthesis of silver nanoparticles using Bacillus megaterium against malarial and dengue vector (Diptera: Culicidae). Parasitol. Res. 2015, 114, 4069–4079. [Google Scholar] [CrossRef]
- Suganya, G.; Karthi, S.; Shivakumar, M.S. Larvicidal potential of silver nanoparticles synthesized from Leucas aspera leaf extracts against dengue vector Aedes aegypti. Parasitol. Res. 2014, 113, 875–880. [Google Scholar] [CrossRef]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, E.; Zhang, M.; Zhang, Y.; Hossain, A.; Qiu, W.; Chen, Y.; Wang, Y.; Wu, W.; Sun, G.; Li, B. Green-Synthesization of Silver Nanoparticles Using Endophytic Bacteria Isolated from Garlic and Its Antifungal Activity against Wheat Fusarium Head Blight Pathogen Fusarium graminearum. Nanomaterials 2020, 10, 219. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10020219
Ibrahim E, Zhang M, Zhang Y, Hossain A, Qiu W, Chen Y, Wang Y, Wu W, Sun G, Li B. Green-Synthesization of Silver Nanoparticles Using Endophytic Bacteria Isolated from Garlic and Its Antifungal Activity against Wheat Fusarium Head Blight Pathogen Fusarium graminearum. Nanomaterials. 2020; 10(2):219. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10020219
Chicago/Turabian StyleIbrahim, Ezzeldin, Muchen Zhang, Yang Zhang, Afsana Hossain, Wen Qiu, Yun Chen, Yanli Wang, Wenge Wu, Guochang Sun, and Bin Li. 2020. "Green-Synthesization of Silver Nanoparticles Using Endophytic Bacteria Isolated from Garlic and Its Antifungal Activity against Wheat Fusarium Head Blight Pathogen Fusarium graminearum" Nanomaterials 10, no. 2: 219. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10020219