Comparison between Citral and Pompia Essential Oil Loaded in Phospholipid Vesicles for the Treatment of Skin and Mucosal Infections
Abstract
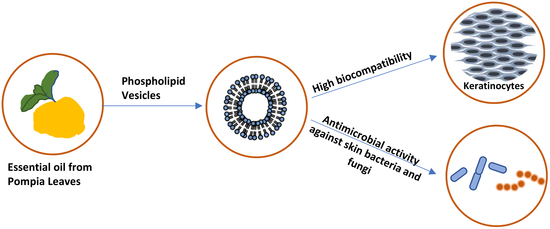
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Antimicrobial Agents
2.3. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.4. Vesicle Preparation
2.5. Vesicle Characterisation
2.6. In Vitro Biocompatibility
2.7. Microbial Strains
2.8. Antimicrobial Tests
2.8.1. Agar Diffusion Method
2.8.2. Micro-Dilution Method
2.9. Statistical Data Analysis
3. Results
3.1. Essential Oil Characterisation
3.2. Vesicle Preparation and Characterisation
3.3. In Vitro Biocompatibility Studies in Keratinocytes
3.4. Agar Diffusion Method
3.5. Micro-Dilution Method
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Camarda, I.; Mazzola, P.; Brunu, A.; Fenu, G.; Lombardo, G.; Palla, F. Un Agrume Nella Storia Della Sardegna: Citrus Limon Var. Pompia Camarda Var. Nova. Quad. Bot. Amb. Appl. 2013, 24, 109–118. [Google Scholar]
- Manconi, M.; Manca, M.L.; Marongiu, F.; Caddeo, C.; Castangia, I.; Petretto, G.L.; Pintore, G.; Sarais, G.; D’hallewin, G.; Zaru, M.; et al. Chemical Characterization of Citrus Limon Var. Pompia and Incorporation in Phospholipid Vesicles for Skin Delivery. Int. J. Pharm. 2016, 506, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Fancello, F.; Petretto, G.L.; Zara, S.; Sanna, M.L.; Addis, R.; Maldini, M.; Foddai, M.; Rourke, J.P.; Chessa, M.; Pintore, G. Chemical Characterization, Antioxidant Capacity and Antimicrobial Activity Against Food Related Microorganisms of Citrus Limon Var. Pompia Leaf Essential Oil. LWT-Food Sci. Technol. 2016, 69, 579–585. [Google Scholar] [CrossRef]
- Fraternale, D.; Giamperi, L.; Bucchini, A.; Cara, P.; Ricci, D. In Vitro Plant Regeneration from Callus of Citrus X Monstruosa (Pompia), an Endemic Citrus of Sardinia. Nat. Prod. Commun. 2010, 5, 927–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saddiq, A.A.; Khayyat, S.A. Chemical and Antimicrobial Studies of Monoterpene: Citral. Pestic. Biochem. Physiol. 2010, 98, 89–93. [Google Scholar] [CrossRef]
- Martins, A.P.; Salgueiro, L.; Goncalves, M.J.; da Cunha, A.P.; Vila, R.; Canigueral, S.; Mazzoni, V.; Tomi, F.; Casanova, J. Essential Oil Composition and Antimicrobial Activity of Three Zingiberaceae from S. Tome E Principe. Planta Med. 2001, 67, 580–584. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, J.; Chen, H.; Song, Z.; Guo, H.; Yuan, Y.; Yue, T. Antibacterial Activity of Essential Oils Against Stenotrophomonas Maltophilia and the Effect of Citral on Cell Membrane. LWT 2020, 117, 108667. [Google Scholar] [CrossRef]
- Hu, L.; Du, M.; Zhang, J.; Wang, Y. Chemistry of the Main Component of Essential Oil of Litsea Cubeba and its Derivatives. Open J. For. 2014, 4, 457–466. [Google Scholar]
- Hu, W.; Li, C.; Dai, J.; Cui, H.; Lin, L. Antibacterial Activity and Mechanism of Litsea Cubeba Essential Oil Against Methicillin-Resistant Staphylococcus Aureus (MRSA). Ind. Crops Prod. 2019, 130, 34–41. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Koning, G.A.; Storm, G. Targeted Drug Delivery Systems for the Intracellular Delivery of Macromolecular Drugs. Drug Discov. Today 2003, 8, 482–483. [Google Scholar] [CrossRef]
- Metselaar, J.M.; Storm, G. Liposomes in the Treatment of Inflammatory Disorders. Expert. Opin. Drug Deliv. 2005, 2, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.S.; Dziubla, T.; Shuvaev, V.V.; Muro, S.; Muzykantov, V.R. Advanced Drug Delivery Systems that Target the Vascular Endothelium. Mol. Interv. 2006, 6, 98–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, S.; Wu, S.Y. The use of Lipid-Based Nanocarriers for Targeted Pain Therapies. Front. Pharmacol. 2013, 4, 143. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Li, W.; Li, C.; Vittayapadung, S.; Lin, L. Liposome Containing Cinnamon Oil with Antibacterial Activity Against Methicillin-Resistant Staphylococcus Aureus Biofilm. Biofouling 2016, 32, 215–225. [Google Scholar] [CrossRef]
- Manconi, M.; Petretto, G.; D’hallewin, G.; Escribano, E.; Milia, E.; Pinna, R.; Palmieri, A.; Firoznezhad, M.; Peris, J.E.; Usach, I.; et al. Thymus Essential Oil Extraction, Characterization and Incorporation in Phospholipid Vesicles for the Antioxidant/Antibacterial Treatment of Oral Cavity Diseases. Colloids Surf. B Biointerfaces 2018, 171, 115–122. [Google Scholar] [CrossRef]
- Risaliti, L.; Kehagia, A.; Daoultzi, E.; Lazari, D.; Bergonzi, M.C.; Vergkizi-Nikolakaki, S.; Hadjipavlou-Litina, D.; Bilia, A.R. Liposomes Loaded with Salvia Triloba and Rosmarinus Officinalis Essential Oils: In Vitro Assessment of Antioxidant, Antiinflammatory and Antibacterial Activities. J. Drug Deliv. Sci. Technol. 2019, 51, 493–498. [Google Scholar] [CrossRef]
- Castangia, I.; Caddeo, C.; Manca, M.L.; Casu, L.; Latorre, A.C.; Diez-Sales, O.; Ruiz-Sauri, A.; Bacchetta, G.; Fadda, A.M.; Manconi, M. Delivery of Liquorice Extract by Liposomes and Hyalurosomes to Protect the Skin Against Oxidative Stress Injuries. Carbohydr. Polym. 2015, 134, 657–663. [Google Scholar] [CrossRef]
- Manca, M.L.; Castangia, I.; Zaru, M.; Nacher, A.; Valenti, D.; Fernandez-Busquets, X.; Fadda, A.M.; Manconi, M. Development of Curcumin Loaded Sodium Hyaluronate Immobilized Vesicles (Hyalurosomes) and their Potential on Skin Inflammation and Wound Restoring. Biomaterials 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Manca, M.L.; Manconi, M.; Falchi, A.M.; Castangia, I.; Valenti, D.; Lampis, S.; Fadda, A.M. Close-Packed Vesicles for Diclofenac Skin Delivery and Fibroblast Targeting. Colloids Surf. B Biointerfaces 2013, 111, 609–617. [Google Scholar] [CrossRef]
- Ihaka, R.; Gentleman, R. R: A Language for Data Analysis and Graphics. J. Comput. Gr. Stat. 1996, 5, 299–314. [Google Scholar]
- Sherry, M.; Charcosset, C.; Fessi, H.; Greige-Gerges, H. Essential Oils Encapsulated in Liposomes: A Review. J. Liposome Res. 2013, 23, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Castangia, I.; Manca, M.L.; Caddeo, C.; Maxia, A.; Murgia, S.; Pons, R.; Demurtas, D.; Pando, D.; Falconieri, D.; Peris, J.E. Faceted Phospholipid Vesicles Tailored for the Delivery of Santolina Insularis Essential Oil to the Skin. Colloids Surf. B Biointerfaces 2015, 132, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Z.; Liu, B.; Zheng, Z.; You, X.; Pu, Y.; Li, Q. Preparation of Liposomes Entrapping Essential Oil from Atractylodes Macrocephala Koidz by Modified RESS Technique. Chem. Eng. Res. Des. 2010, 88, 1102–1107. [Google Scholar] [CrossRef]
- Espina, L.; Berdejo, D.; Alfonso, P.; García-Gonzalo, D.; Pagán, R. Potential use of Carvacrol and Citral to Inactivate Biofilm Cells and Eliminate Biofouling. Food Control 2017, 82, 256–265. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossiter, S.E.; Fletcher, M.H.; Wuest, W.M. Natural Products as Platforms to Overcome Antibiotic Resistance. Chem. Rev. 2017, 117, 12415–12474. [Google Scholar] [CrossRef]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole Plant Extracts Versus Single Compounds for the Treatment of Malaria: Synergy and Positive Interactions. Malar. J. 2011, 10, S4. [Google Scholar] [CrossRef] [Green Version]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar]
- Kohlert, C.; van Rensen, I.; Marz, R.; Schindler, G.; Graefe, E.U.; Veit, M. Bioavailability and Pharmacokinetics of Natural Volatile Terpenes in Animals and Humans. Planta Med. 2000, 66, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Mechanisms of Membrane Toxicity of Hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, K.; Pauli, A.; Iberl, B.; Weigand, H.; Weis, N. Antibacterial and Antifungal Properties of Essential Oil Components. J. Essent. Oil Res. 1989, 1, 119–128. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, X.; Li, F. Advances in Pharmaceutical Studies on Improvement of Stability of Volatile Oils of Chinese Materia Medica. Pharm. Care Res. 2008, 8, 197–200. [Google Scholar]
- Zhao, Y.; Wang, C.; Chow, A.H.; Ren, K.; Gong, T.; Zhang, Z.; Zheng, Y. Self-Nanoemulsifying Drug Delivery System (SNEDDS) for Oral Delivery of Zedoary Essential Oil: Formulation and Bioavailability Studies. Int. J. Pharm. 2010, 383, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Manconi, M.; Aparicio, J.; Vila, A.O.; Pendás, J.; Figueruelo, J.; Molina, F. Viscoelastic Properties of Concentrated Dispersions in Water of Soy Lecithin. Colloids Surf. A Physicochem. Eng. Asp. 2003, 222, 141–145. [Google Scholar] [CrossRef]
- De Matos, S.P.; Teixeira, H.F.; De Lima, A.A.N.; Veiga-Junior, V.F.; Koester, L.S. Essential Oils and Isolated Terpenes in Nanosystems Designed for Topical Administration: A Review. Biomolecules 2019, 9, 138. [Google Scholar] [CrossRef] [Green Version]
- Egbaria, K.; Weiner, N. Liposomes as a Topical Drug Delivery System. Adv. Drug Deliv. Rev. 1990, 5, 287–300. [Google Scholar] [CrossRef] [Green Version]
- Manconi, M.; Pendas, J.; Ledon, N.; Moreira, T.; Sinico, C.; Saso, L.; Fadda, A.M. Phycocyanin Liposomes for Topical Anti-Inflammatory Activity: In-Vitro in-Vivo Studies. J. Pharm. Pharmacol. 2009, 61, 423–430. [Google Scholar] [CrossRef]
- Singh, M.; Devi, S.; Rana, V.S.; Mishra, B.B.; Kumar, J.; Ahluwalia, V. Delivery of Phytochemicals by Liposome Cargos: Recent Progress, Challenges and Opportunities. J. Microencapsul. 2019, 36, 215–235. [Google Scholar] [CrossRef]
- Hammoud, Z.; Gharib, R.; Fourmentin, S.; Elaissari, A.; Greige-Gerges, H. New Findings on the Incorporation of Essential Oil Components into Liposomes Composed of Lipoid S100 and Cholesterol. Int. J. Pharm. 2019, 561, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Castangia, I.; Manca, M.L.; Caddeo, C.; Bacchetta, G.; Pons, R.; Demurtas, D.; Diez-Sales, O.; Fadda, A.M.; Manconi, M. Santosomes as Natural and Efficient Carriers for the Improvement of Phycocyanin Reepithelising Ability in Vitro and in Vivo. Eur. J. Pharm. Biopharm. 2016, 103, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Ezzeddine, N.B.; Abdelkéfi, M.M.; Aissa, R.B.; Chaabouni, M.M. Antibacterial Screening of Origanum Majorana L. Oil from Tunisia. J. Essent. Oil Res. 2001, 13, 295–297. [Google Scholar] [CrossRef]


| Compound | RT | A% | RI |
|---|---|---|---|
| α-Pinene | 13.37 | 0.4 | 1033 |
| Camphene | 14.85 | tr | 1081 |
| β-Pinene | 16.26 | 1.1 | 1126 |
| Sabinene | 16.67 | 0.4 | 1139 |
| 3-carene | 17.59 | 0.9 | 1166 |
| β-Myrcene | 17.87 | 0.8 | 1175 |
| 2,3-Dehydro-1,8-cineole | 18.89 | tr | 1206 |
| limonene | 19.24 | 29.7 | 1217 |
| β-Z-Ocimene | 20.26 | 0.5 | 1249 |
| β-E-Ocimene | 20.83 | 4.4 | 1267 |
| p-Cymene | 21.55 | 0.7 | 1290 |
| Terpinolene | 21.99 | tr | 1304 |
| 5-Hepten-2-one, 6-methyl- | 23.43 | 0.9 | 1352 |
| cis-Linalool oxide | 26.27 | 0.1 | 1448 |
| trans-Linalool oxide (furanoid) | 27.05 | 0.0 | 1475 |
| Citronellal | 27.32 | 0.2 | 1484 |
| Linalool | 28.87 | 11.0 | 1535 |
| Linalyl acetate | 29.46 | 20.9 | 1555 |
| 3-Methoxy-p-cymene | 30.73 | 0.2 | 1596 |
| Terpinen-4-ol | 31.07 | 0.3 | 1609 |
| Megastigma-triene (not identified isomer) | 31.40 | 0.7 | 1622 |
| Neral | 33.07 | 6.8 | 1689 |
| α-Terpineol | 33.17 | 2.2 | 1693 |
| Neryl acetate | 33.72 | 1.1 | 1718 |
| Geranial | 34.06 | 11.1 | 1735 |
| Nerol | 34.29 | 2.9 | 1746 |
| cis-geraniol | 35.00 | 0.5 | 1780 |
| Geraniol | 35.74 | 1.2 | 1819 |
| Unknown | 38.48 | 0.3 | 1985 |
| Unknown | 38.59 | 0.2 | 1992 |
| Unknown | 40.12 | 0.2 | 2090 |
| Menthadien-1,2-diol | 41.87 | 0.1 | 2216 |
| Neric acid | 42.90 | 0.2 | 2286 |
| MD (nm) | PI | ZP (mV) | EE (%) | |
|---|---|---|---|---|
| Empty liposomes | 75 ± 4 | 0.28 ± 0.02 | −70 ± 2 | - |
| Pompia e.o. loaded liposomes | 152 ± 18 | 0.31 ± 0.05 | −74 ± 5 | 85 ± 15 |
| Citral loaded liposomes | 129 ± 16 | 0.32 ± 0.05 | −72 ± 4 | 88 ± 13 |
| Compound (concentration) | E. coli IH (mm) | P. aeruginosa IH (mm) | S. aureus IH (mm) | C. albicans IH (mm) |
|---|---|---|---|---|
| Pompia e.o. (300 mg/mL) | 13 ± 2 | <5 | 25 ± 6 | 16 ± 3 |
| Citral (300 mg/mL) | 20 ± 4 | <5 | 37 ± 1 | 25 ± 5 |
| Gentamicin (1.5 mg/mL) | 22 ± 4 | 24 ± 2 | 27 ± 2 | - |
| Clotrimazole (250 µg/mL) | - | - | - | 25 ± 1 |
| MIC50 (mg/mL) | MFC or MBC | |
|---|---|---|
| E. coli | ||
| Pompia e.o. | 3 | 12 |
| Pompia e.o. loaded liposomes | 10 | >10 |
| Citral | 1.5 | 6 |
| Citral loaded liposomes | 5 | 10 |
| P. aeruginosa | ||
| Pompia e.o. | 6 | 12 |
| Pompia e.o. loaded liposomes | 5 | 10 |
| Citral | 6 | >12 |
| Citral loaded liposomes | 5 | 10 |
| S. aureus | ||
| Pompia e.o. | 3 | 6 |
| Pompia e.o. loaded liposomes | 10 | >10 |
| Citral | 1.5 | 3 |
| Citral loaded liposomes | 5 | 5 |
| C. albicans | ||
| Pompia e.o. | 3 | 6 |
| Pompia e.o. loaded liposomes | 2.5 | 10 |
| Citral | 1.5 | 3 |
| Citral loaded liposomes | 0.6 | 2.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usach, I.; Margarucci, E.; Manca, M.L.; Caddeo, C.; Aroffu, M.; Petretto, G.L.; Manconi, M.; Peris, J.-E. Comparison between Citral and Pompia Essential Oil Loaded in Phospholipid Vesicles for the Treatment of Skin and Mucosal Infections. Nanomaterials 2020, 10, 286. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10020286
Usach I, Margarucci E, Manca ML, Caddeo C, Aroffu M, Petretto GL, Manconi M, Peris J-E. Comparison between Citral and Pompia Essential Oil Loaded in Phospholipid Vesicles for the Treatment of Skin and Mucosal Infections. Nanomaterials. 2020; 10(2):286. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10020286
Chicago/Turabian StyleUsach, Iris, Elisabetta Margarucci, Maria Letizia Manca, Carla Caddeo, Matteo Aroffu, Giacomo L. Petretto, Maria Manconi, and José-Esteban Peris. 2020. "Comparison between Citral and Pompia Essential Oil Loaded in Phospholipid Vesicles for the Treatment of Skin and Mucosal Infections" Nanomaterials 10, no. 2: 286. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10020286