Ex Vivo Permeation of Carprofen Vehiculated by PLGA Nanoparticles through Porcine Mucous Membranes and Ophthalmic Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
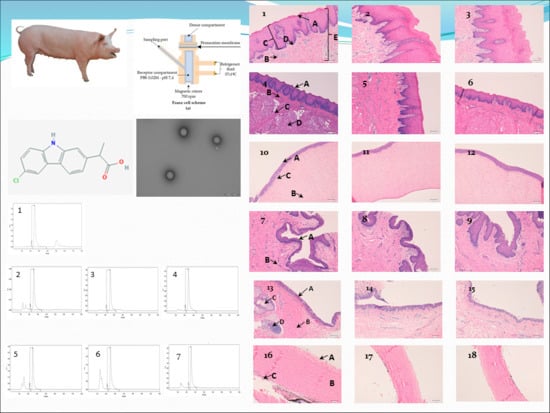
2.2. NPs of Carprofen
2.2.1. Materials of NPs
2.2.2. Preparation of NPs
2.2.3. Physicochemical Characterization
2.3. Permeation Studies
2.3.1. Mucous Membranes
2.3.2. Ex Vivo Study: Franz Diffusion Cells
2.3.3. HPLC–UV
2.3.4. Permeation Parameters
2.3.5. Statistical Analysis
2.4. In Vivo Studies
Determination of the Amount of Drug Remaining in the Mucous Membrane
3. Results
3.1. Permeation Studies
3.2. In Vivo Studies
4. Discussion
4.1. HPLC Results
4.2. Ophthalmic Tissues
4.3. Mucous Membranes
4.4. In Vivo Studies
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yü, T.-F.; Perel, J. Pharmacokinetic and Clinical Studies of Carprofen in Gout. J. Clin. Pharmacol. 1980, 20, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Griswold, D.E.; Adams, J.L. Constitutive cyclooxygenase (COX-1) and inducible cyclooxygenase (COX-2): Rationale for selective inhibition and progress to date. Med. Res. Rev. 1996, 16, 181–206. [Google Scholar] [CrossRef]
- Kerr, A.C.; Muller, F.; Ferguson, J.; Dawe, R.S. Occupational carprofen photoallergic contact dermatitis. Br. J. Dermatol. 2008, 159, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Committe for Veterinary Medicinal Products Carpronfen Summary Report; EMEA/MRL/042/95-FINAL; The European Agency for the Evaluation of Medicinal Products: London, UK, 1999.
- Armstrong, S.; Tricklebank, P.; Lake, A.; Frean, S.; Lees, P. Pharmacokinetics of carprofen enantiomers in equine plasma and synovial fluid—A comparison with ketoprofen. J. Vet. Pharmacol. Ther. 1999, 22, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Lees, P.; Landoni, M.F.; Ciraudel, J.; Toutain, P.L. Pharmacodynamics and pharmacokinetics of nonsteroidal anti-inflammatory drugs in species of veterinary interest. J. Vet. Pharmacol. Ther. 2004, 27, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Lascelles, B.D.X.; Cripps, P.J.; Jones, A.; Waterman-Pearson, A.E. Efficacy and kinetics of carprofen, administered preoperatively or postoperatively, for the prevention of pain in dogs undergoing ovariohysterectomy. Vet. Surg. 1998, 27, 568–582. [Google Scholar] [CrossRef]
- Grisneaux, E.; Pibarot, P.; Dupuis, J.; Biais, D. Comparison of ketoprofen and carprofen administered prior to orthopedic surgery for control of postoperative pain in dogs. J. Am. Vet. Med Assoc. 1999, 215, 1105–1110. [Google Scholar]
- Slingsby, L.S.; Waterman-Pearson, A.E. Analgesic effects in dogs of carprofen and pethidine together compared with the effects of either drug alone. Vet. Rec. 2001, 148, 441–444. [Google Scholar] [CrossRef]
- Slingsby, L.S.; Waterman-Pearson, A.E. Comparison between meloxicam and carprofen for postoperative analgesia after feline ovariohysterectomy. J. Small Anim. Pract. 2002, 43, 286–289. [Google Scholar] [CrossRef]
- McGeown, D.; Danbury, T.C.; Waterman-Pearson, A.E.; Kestin, S.C. Effect of carprofen on lameness in broiler chickens. Vet. Rec. 1999, 144, 668–671. [Google Scholar] [CrossRef]
- Roughan, J.V.; Flecknell, P.A. Behaviour-based assessment of the duration of laparotomy-induced abdominal pain and the analgesic effects of carprofen and buprenorphine in rats. Behav. Pharmacol. 2004, 15, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, N.V.; Simmons, D.L. The cyclooxygenases. Genome Biol. 2004, 5, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Drug Administration. FDA Approved Animal Drug Products Database. Available online: https://animaldrugsatfda.fda.gov/adafda/views/#/home/searchResult (accessed on 13 June 2019).
- Sanderson, R.O.; Beata, C.; Flipo, R.M.; Genevois, J.P.; Macias, C.; Tacke, S.; Innes, J.F. Systematic review of the management of canine osteoarthritis. Vet. Rec. 2009, 164, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Slingsby, L.S.; Jones, A.; Waterman-Pearson, A.E. Use of a new finger-mounted device to compare mechanical nociceptive thresholds in cats given pethidine or no medication after castration. Res. Vet. Sci. 2001, 70, 243–246. [Google Scholar] [CrossRef]
- Bergmann, H.M.; Nolte, I.; Kramer, S. Comparison of analgesic efficacy of preoperative or postoperative carprofen with or without preincisional mepivacaine epidural anesthesia in canine pelvic or femoral fracture repair. Vet. Surg. 2007, 36, 623–632. [Google Scholar] [CrossRef]
- Sidler, M.; Fouché, N.; Meth, I.; von Hahn, F.; von Rechenberg, B.; Kronen, P. Transcutaneous Treatment with Vetdrop® Sustains the Adjacent Cartilage in a Microfracturing Joint Defect Model in Sheep. Open Orthop. J. 2013, 7, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Elitok, B.; Elitok, Ö.M. Clinical efficacy of carprofen as an adjunct to the antibacterial treatment of bovine respiratory disease. J. Vet. Pharmacol. Ther. 2004, 27, 317–320. [Google Scholar] [CrossRef]
- Lockwood, P.W.; Johnson, J.C.; Katz, T.L. Clinical efficacy of flunixin, carprofen and ketoprofen as adjuncts to the antibacterial treatment of bovine respiratory disease. Vet. Rec. 2003, 152, 392–394. [Google Scholar] [CrossRef]
- Mathews, K.A. Non-steroidal anti-inflammatory analgesics for acute pain management in dogs and cats. Vet. Comp. Orthop. Traumatol. 1997, 10, 122–129. [Google Scholar] [CrossRef]
- Mcpherson, M.L.; Cimino, N.M. Topical NSAID formulations. Pain Med. (U.S.) 2013, 14, S35–S39. [Google Scholar] [CrossRef]
- Amores, S.; Domenech, J.; Colom, H.; Calpena, A.C.; Clares, B.; Gimeno, Á.; Lauroba, J. An improved cryopreservation method for porcine buccal mucous in Ex Vivo drug permeation studies using Franz diffusion cells. Eur. J. Pharm. Sci. 2014, 60, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Alqurshi, A.; Hanafy, A.F.; Abdalla, A.M.; Guda, T.K.; Gabr, K.E.; Royall, P.G. Ocular anti-inflammatory activity of prednisolone acetate loaded chitosan-deoxycholate self-assembled nanoparticles. Int. J. Nanomed. 2019, 14, 3679–3689. [Google Scholar]
- Perpiñán, D.; Bargalló, F.; Grifols, J. Correction of periocular fat pad hypertrophy and entropion in a pot-bellied pig (Sus scrofa). Clínica Vet. Pequeños Anim. 2015, 35, 27–30. [Google Scholar]
- Moreau, M.E.; Dubreuil, P.; Molinaro, G.; Chagnon, M.; Müller-Esterl, W.; Lepage, Y.; Adam, A. Expression of metallopeptidases and kinin receptors in swine oropharyngeal tissues: Effects of angiotensin I-converting enzyme inhibition and inflammation. J. Pharmacol. Exp. Ther. 2005, 315, 1065–1074. [Google Scholar] [CrossRef] [Green Version]
- Takalkar, D.; Desai, N. Nanolipid Gel of an Antimycotic Drug for Treating Vulvovaginal Candidiasis—Development and Evaluation. AAPS PharmSciTech 2018, 19, 1297–1307. [Google Scholar] [CrossRef]
- Newell-Fugate, A.E.; Lenz, K.; Skenandore, C.; Nowak, R.A.; White, B.A.; Braundmeier-Fleming, A. Effects of coconut oil on glycemia, inflammation, and urogenital microbial parameters in female Ossabaw mini-pigs. PLoS ONE 2017, 12, e0179542. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Bell, M.; O’Connor, C.; Inchley, A.; Wibawa, J.; Lane, M.E. Delivery of ibuprofen to the skin. Int. J. Pharm. 2013, 457, 9–13. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Fang, L.; Tan, Z.; Wu, J.; He, Z.G. Influence of ion-pairing and chemical enhancers on the transdermal delivery of meloxicam Enhanced penetration of meloxicam. Drug Dev. Ind. Pharm. 2009, 35, 663–670. [Google Scholar] [CrossRef]
- Singh, P.; Roberts, M.S. Skin permeability and local tissue concentrations of nonsteroidal anti- inflammatory drugs after topical application. J. Pharmacol. Exp. Ther. 1994, 268, 144–151. [Google Scholar]
- Parra, A.; Clares, B.; Rosselló, A.; Garduño-Ramírez, M.L.; Abrego, G.; García, M.L.; Calpena, A.C. Ex Vivo permeation of carprofen from nanoparticles: A comprehensive study through human, porcine and bovine skin as anti-inflammatory agent. Int. J. Pharm. 2016, 501, 10–17. [Google Scholar] [CrossRef]
- Flaten, G.E.; Palac, Z.; Engesland, A.; Filipović-Grčić, J.; Vanić, Ž.; Škalko-Basnet, N. In vitro skin models as a tool in optimization of drug formulation. Eur. J. Pharm. Sci. 2015, 75, 10–24. [Google Scholar] [CrossRef] [Green Version]
- Bolzinger, M.A.; Briançon, S.; Pelletier, J.; Chevalier, Y. Penetration of drugs through skin, a complex rate-controlling membrane. Curr. Opin. Colloid Interface Sci. 2012, 17, 156–165. [Google Scholar] [CrossRef]
- Magnusson, B.M.; Walters, K.A.; Roberts, M.S. Veterinary drug delivery: Potential for skin penetration enhancement. Adv. Drug Deliv. Rev. 2001, 50, 205–227. [Google Scholar] [CrossRef]
- Parra, A.; Mallandrich, M.; Clares, B.; Egea, M.A.; Espina, M.; García, M.L.; Calpena, A.C. Design and elaboration of freeze-dried PLGA nanoparticles for the transcorneal permeation of carprofen: Ocular anti-inflammatory applications. Colloids Surf. B Biointerfaces 2015, 136, 935–943. [Google Scholar] [CrossRef]
- Zahr, A.S.; De Villiers, M.; Pishko, M.V. Encapsulation of drug nanoparticles in self-assembled macromolecular nanoshells. Langmuir 2005, 21, 403–410. [Google Scholar] [CrossRef]
- Brugués, A.P.; Naveros, B.C.; Calpena Campmany, A.C.; Pastor, P.H.; Saladrigas, R.F.; Lizandra, C.R. Developing cutaneous applications of paromomycin entrapped in stimuli-sensitive block copolymer nanogel dispersions. Nanomedicine 2015, 10, 227–240. [Google Scholar] [CrossRef]
- Netzlaff, F.; Schaefer, U.F.; Lehr, C.M.; Meiers, P.; Stahl, J.; Kietzmann, M.; Niedorf, F. Comparison of bovine udder skin with human and porcine skin in percutaneous permeation experiments. Atla Altern. Lab. Anim. 2006, 34, 499–513. [Google Scholar]
- Seto, J.E.; Polat, B.E.; Lopez, R.F.V.; Blankschtein, D.; Langer, R. Effects of ultrasound and sodium lauryl sulfate on the transdermal delivery of hydrophilic permeants: Comparative in vitro studies with full-thickness and split-thickness pig and human skin. J. Control. Release 2010, 145, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Cañadas-Enrich, C.; Abrego, G.; Alvarado, H.L.; Calpena-Campmany, A.C.; Boix-Montañes, A. Pranoprofen quantification in Ex Vivo corneal and scleral permeation samples: Analytical validation. J. Pharm. Biomed. Anal. 2018, 160, 109–118. [Google Scholar] [CrossRef]
- Sanz, R.; Calpena, A.C.; Mallandrich, M.; Gimeno, Á.; Halbaut, L.; Clares, B. Development of a buccal doxepin platform for pain in oral mucositis derived from head and neck cancer treatment. Eur. J. Pharm. Biopharm. 2017, 117, 203–211. [Google Scholar] [CrossRef]
- Williams, A.C.; Cornwell, P.A.; Barry, B.W. On the non-Gaussian distribution of human skin permeabilities. Int. J. Pharm. 1992, 86, 69–77. [Google Scholar] [CrossRef]
- Raber, A.S.; Mittal, A.; Schäfer, J.; Bakowsky, U.; Reichrath, J.; Vogt, T.; Schaefer, U.F.; Hansen, S.; Lehr, C.M. Quantification of nanoparticle uptake into hair follicles in pig ear and human forearm. J. Control. Release 2014, 179, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, P.; Morais, S.; Carmo-Pereira, M. Nanomaterials towards Biosensing of Alzheimer’s Disease Biomarkers. Nanomaterials 2019, 9, 1663. [Google Scholar] [CrossRef] [Green Version]
- Bamrungsap, S.; Zhao, Z.; Chen, T.; Wang, L.; Li, C.; Fu, T.; Tan, W. Nanotechnology in therapeutics: A focus on nanoparticles as a drug delivery system. Nanomedicine 2012, 7, 1953–1971. [Google Scholar] [CrossRef]
- Bacha, W.J., Jr.; Bacha, L.M. Color Atlas of Veterinary Histology, 3rd ed.; Wiley&BlackWell: New York, NY, USA, 2012; pp. 122–188. [Google Scholar]
- Zimmerman, J.J.; Karriker, L.A.; Ramirez, A.; Schwartz, K.J.; Stevenson, G.W.; Zhang, J. Diseases of Swine, 11th ed.; Wiley&BlackWell: New York, NY, USA, 2019; pp. 292–392. [Google Scholar]













| Type of Tissue | Retention Time (Minutes) |
|---|---|
| CP validation line (100 µg/mL) | 2.516 |
| Sclera | 2.54 |
| Cornea | 2.574 |
| Conjunctiva | 2.445 |
| Buccal mucous | 2.539 |
| Sublingual mucous | 2.501 |
| Vaginal mucous | 2.531 |
| CP-Solution | CP-NPs | |||||
|---|---|---|---|---|---|---|
| Conjunctiva | Cornea | Sclera | Conjunctiva | Cornea | Sclera | |
| Js (mcg/h) | 9.4 | 5.08 | 0.9 | 4.19 ** | 1.24 | 1.01 |
| (8.50–10.20) | (1.13–6.16) | (0.22–1.58) | (0.50–7.88) | (0.42–4.73) | (0.96–1.05) | |
| Tl (h) | 1.45 | 1.71 | 2.04 | 0.31 ** | 1.72 | 2.7 ** |
| (1.42–1.45) | (1.51–2.33) | (1.86–2.22) | (0.19–1.43) | (1.31–2.44) | (2.66–2.73) | |
| P2 × 101 (h–1) | 1.15 | 0.98 | 0.82 | 6.32 ** | 0.97 | 0.62 ** |
| (0.84–1.47) | (0.72–1.10) | (0.75–0.90) | (3.88–8.77) | (0.68–1.27) | (0.61–0.63) | |
| P1 × 102 (cm) | 17.01 | 11.91 | 2.13 | 2.18 ** | 3.34 | 3.39 |
| (15.80–20.0) | (2.14–15.38) | (0.60–3.66) | (0.12–4.24) | (0.69–9.37) | (3.27–3.50) | |
| Kp × 103 (cm·h) | 19.58 | 10.58 | 1.87 | 8.73 * | 2.58 | 2.09 |
| (15.40–22.0) | (2.36–12.83) | (0.45–3.29) | (1.04–16.41) | (0.88–9.85) | (2.0–2.19) | |
| Qr (mcg/cm2/g) | 3.62 | 16.56 | 12.21 | 3.57 | 20.89 ** | 12.25 |
| (3.61–3.63) | (16.10–17.06) | (12.17–12.26) | (2.89–4.25) | (18.55–23.23) | (12.16–12.34) | |
| CP-Solution | CP-NPs | |||||
|---|---|---|---|---|---|---|
| Buccal | Sublingual | Vaginal | Buccal | Sublingual | Vaginal | |
| Js (mcg/h) | 0.74 | 4.81 | 3.91 | 2.76 ** | 0.31 ** | 8.89 ** |
| (0.73–0.75) | (1.37–8.24) | (3.83–3.99) | (1.70–3.82) | (0.14–0.48) | (5.09–12.69) | |
| Tl (h) | 1.65 | 2.77 | 3.34 | 0.8 ** | 2.09 ** | 1.75 ** |
| (1.5–1.80) | (2.68–2.87) | (2.57–4.11) | (0.74–0.86) | (1.53–2.65) | (1.4–2.1) | |
| P2 × 101 (h−1) | 1.02 | 0.6 | 0.53 | 2.1 ** | 0.86 ** | 0.99 ** |
| (0.92–1.11) | (0.58–0.62) | (0.41–0.65) | (1.94–2.25) | (0.63–1.09) | (0.79–1.19) | |
| P1 × 102 (cm) | 0.38 | 16.24 | 16.25 | 0.72* | 0.93 ** | 17.78 |
| (0.35–0.41) | (4.91–27.56) | (12.82–29.67) | (0.40–1.04) | (0.27–1.60) | (13.35–22.21) | |
| Kp × 103 (cm·h) | 0.39 | 10.01 | 8.15 | 1.45 ** | 0.65 ** | 18.52 ** |
| (0.38–0.39) | (2.86–17.17) | (7.99–8.31) | (0.89–2.01) | (0.30–1.0) | (10.59–26.44) | |
| Qr (mcg/cm2/g) | 2.38 | 33.14 | 23.44 | 3.46 * | 29.13 ** | 52.81 ** |
| (2.2–2.56) | (33.09–33.18) | (20.7–26.19) | (2.49–3.63) | (27.81–30.,45) | (49.41–56.21) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Segura, L.; Parra, A.; Calpena-Campmany, A.C.; Gimeno, Á.; Gómez de Aranda, I.; Boix-Montañes, A. Ex Vivo Permeation of Carprofen Vehiculated by PLGA Nanoparticles through Porcine Mucous Membranes and Ophthalmic Tissues. Nanomaterials 2020, 10, 355. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10020355
Gómez-Segura L, Parra A, Calpena-Campmany AC, Gimeno Á, Gómez de Aranda I, Boix-Montañes A. Ex Vivo Permeation of Carprofen Vehiculated by PLGA Nanoparticles through Porcine Mucous Membranes and Ophthalmic Tissues. Nanomaterials. 2020; 10(2):355. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10020355
Chicago/Turabian StyleGómez-Segura, Lídia, Alexander Parra, Ana Cristina Calpena-Campmany, Álvaro Gimeno, Immaculada Gómez de Aranda, and Antonio Boix-Montañes. 2020. "Ex Vivo Permeation of Carprofen Vehiculated by PLGA Nanoparticles through Porcine Mucous Membranes and Ophthalmic Tissues" Nanomaterials 10, no. 2: 355. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10020355