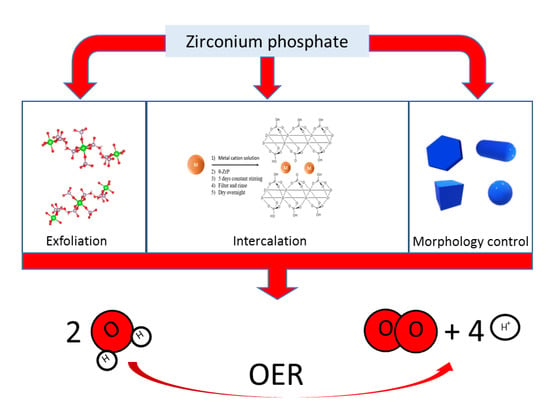
Preparation of Zirconium Phosphate Nanomaterials and Their Applications as Inorganic Supports for the Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Synthesis and Preparation of ZrP Nanomaterials
2.1. α-ZrP
2.2. ZrP Exfoliation
2.3. ZrP with Different Morphologies
2.4. Intercalation of Guest Species into ZrP
3. ZrP Nanomaterials as Support for OER Active Species
3.1. Intercalated and Surface Adsorbed Transition Metals ZrP Electrocatalysts
3.2. Exfoliation of ZrP for Improved OER
3.3. Nanostructuring ZrP to Support Transition Metals Species
4. Conclusions
Funding
Conflicts of Interest
References
- Clearfield, A.; Stynes, J.A. The Preparation of Crystalline Zirconium Phosphate and Some Observations on Its Ion Exchange Behaviour. J. Inorg. Nucl. Chem. 1964, 26, 117–129. [Google Scholar] [CrossRef]
- Troup, J.M.; Clearfield, A. Mechanism of Ion Exchange in Zirconium Phosphates. 20. Refinement of the Crystal Structure of. Alpha.-Zirconium Phosphate. Inorg. Chem. 1977, 16, 3311–3314. [Google Scholar] [CrossRef]
- Clearfield, A.; Smith, G.D. Crystallography and Structure of. Alpha.-Zirconium Bis(Monohydrogen Orthophosphate) Monohydrate. Inorg. Chem. 1969, 8, 431–436. [Google Scholar] [CrossRef]
- Clearfield, A.; Blessing, R.H.; Stynes, J.A. New Crystalline Phases of Zirconium Phosphate Possessing Ion-Exchange Properties. J. Inorg. Nucl. Chem. 1968, 30, 2249–2258. [Google Scholar] [CrossRef]
- Clearfield, A.; Duax, W.L.; Medina, A.S.; Smith, G.D.; Thomas, J.R. Mechanism of Ion Exchange in Crystalline Zirconium Phosphates. I. Sodium Ion Exchange of. Alpha.-Zirconium Phosphate. J. Phys. Chem. 1969, 73, 3424–3430. [Google Scholar] [CrossRef]
- Zhou, Y.; Noshadi, I.; Ding, H.; Liu, J.; Parnas, R.S.; Clearfield, A.; Xiao, M.; Meng, Y.; Sun, L. Solid Acid Catalyst Based on Single-Layer α-Zirconium Phosphate Nanosheets for Biodiesel Production via Esterification. Catalysts 2018, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Pica, M. Zirconium Phosphate Catalysts in the XXI Century: State of the Art from 2010 to Date. Catalysts 2017, 7, 190. [Google Scholar] [CrossRef]
- Cheng, Y.; Chuah, G.K. The Synthesis and Applications of α-Zirconium Phosphate. Chin. Chem. Lett. 2020, 31, 307–310. [Google Scholar] [CrossRef]
- González-Villegas, J.; Kan, Y.; Bakhmutov, V.I.; García-Vargas, A.; Martínez, M.; Clearfield, A.; Colón, J.L. Poly(Ethylene Glycol)-Modified Zirconium Phosphate Nanoplatelets for Improved Doxorubicin Delivery. Inorg. Chim. Acta 2017, 468, 270–279. [Google Scholar] [CrossRef]
- Cruz, E.; Broker, E.J.; Mosby, B.M. Combination of Intercalation and Surface Modification in Layered Zirconium Phosphates: Investigation of Surface Stability and Reactivity. Dalton Trans. 2020. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.; Mosby, B.M.; Bakhmutov, V.I.; Martí, A.A.; Batteas, J.D.; Clearfield, A. Self-Assembled Monolayers Based Upon a Zirconium Phosphate Platform. Chem. Mater. 2013, 25, 723–728. [Google Scholar] [CrossRef]
- Pica, M.; Nocchetti, M.; Ridolfi, B.; Donnadio, A.; Costantino, F.; Gentili, P.L.; Casciola, M. Nanosized Zirconium Phosphate/AgCl Composite Materials: A New Synergy for Efficient Photocatalytic Degradation of Organic Dye Pollutants. J. Mater. Chem. A 2015, 3, 5525–5534. [Google Scholar] [CrossRef]
- Martí, A.A.; Colón, J.L. Direct Ion Exchange of Tris(2,2′-Bipyridine)Ruthenium(II) into an α-Zirconium Phosphate Framework. Inorg. Chem. 2003, 42, 2830–2832. [Google Scholar] [CrossRef] [PubMed]
- Casañas-Montes, B.; Díaz, A.; Barbosa, C.; Ramos, C.; Collazo, C.; Meléndez, E.; Queffelec, C.; Fayon, F.; Clearfield, A.; Bujoli, B.; et al. Molybdocene Dichloride Intercalation into Zirconium Phosphate Nanoparticles. J. Organomet. Chem. 2015, 791, 34–40. [Google Scholar] [CrossRef]
- Díaz, A.; David, A.; Pérez, R.; González, M.L.; Báez, A.; Wark, S.E.; Zhang, P.; Clearfield, A.; Colón, J.L. Nanoencapsulation of Insulin into Zirconium Phosphate for Oral Delivery Applications. Biomacromolecules 2010, 11, 2465–2470. [Google Scholar] [CrossRef] [Green Version]
- Díaz, A.; Saxena, V.; González, J.; David, A.; Casañas, B.; Carpenter, C.; Batteas, J.D.; Colón, J.L.; Clearfield, A.; Hussain, M.D. Zirconium Phosphate Nano-Platelets: A Novel Platform for Drug Delivery in Cancer Therapy. Chem. Commun. 2012, 48, 1754–1756. [Google Scholar] [CrossRef]
- Díaz, A.; González, M.L.; Pérez, R.J.; David, A.; Mukherjee, A.; Báez, A.; Clearfield, A.; Colón, J.L. Direct Intercalation of Cisplatin into Zirconium Phosphate Nanoplatelets for Potential Cancer Nanotherapy. Nanoscale 2013, 5, 11456–11463. [Google Scholar] [CrossRef] [Green Version]
- Santiago, M.B.; Vélez, M.M.; Borrero, S.; Díaz, A.; Casillas, C.A.; Hofmann, C.; Guadalupe, A.R.; Colón, J.L. NADH Electrooxidation Using Bis(1,10-Phenanthroline-5,6-Dione)(2,2′-Bipyridine)Ruthenium(II)-Exchanged Zirconium Phosphate Modified Carbon Paste Electrodes. Electroanalysis 2006, 18, 559–572. [Google Scholar] [CrossRef]
- Santiago, M.B.; Declet-Flores, C.; Díaz, A.; Vélez, M.M.; Bosques, M.Z.; Sanakis, Y.; Colón, J.L. Layered Inorganic Materials as Redox Agents: Ferrocenium-Intercalated Zirconium Phosphate. Langmuir 2007, 23, 7810–7817. [Google Scholar] [CrossRef]
- Santiago, M.B.; Daniel, G.A.; David, A.; Casañas, B.; Hernández, G.; Guadalupe, A.R.; Colón, J.L. Effect of Enzyme and Cofactor Immobilization on the Response of Ethanol Oxidation in Zirconium Phosphate Modified Biosensors. Electroanalysis 2010, 22, 1097–1105. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, R.; Ding, F.; Brittain, A.D.; Liu, J.; Zhang, M.; Xiao, M.; Meng, Y.; Sun, L. Sulfonic Acid-Functionalized α-Zirconium Phosphate Single-Layer Nanosheets as a Strong Solid Acid for Heterogeneous Catalysis Applications. ACS Appl. Mater. Interfaces 2014, 6, 7417–7425. [Google Scholar] [CrossRef]
- Clearfield, A.; Thakur, D.S. Zirconium and Titanium Phosphates as Catalysts: A Review. Appl. Catal. 1986, 26, 1–26. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Karimi, H. Zirconium Phosphate Nanoparticles as a Remarkable Solid Acid Catalyst for Selective Solvent-Free Alkylation of Phenol. Chin. J. Catal. 2014, 35, 1136–1147. [Google Scholar] [CrossRef]
- Niño, M.E.; Giraldo, S.A.; Páez-Mozo, E.A. Olefin Oxidation with Dioxygen Catalyzed by Porphyrins and Phthalocyanines Intercalated in α-Zirconium Phosphate. J. Mol. Catal. Chem. 2001, 175, 139–151. [Google Scholar] [CrossRef]
- Clearfield, A. Group IV Phosphates as Catalysts and Catalyst Supports. J. Mol. Catal. 1984, 27, 251–262. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Karimi, H. Zinc Zirconium Phosphate as an Efficient Catalyst for Chemoselective Synthesis of 1,1-diacetates under Solvent-free Conditions. J. Chem. Sci. 2015, 127, 1945–1955. [Google Scholar] [CrossRef] [Green Version]
- Alongi, J.; Frache, A. Flame Retardancy Properties of α-Zirconium Phosphate Based Composites. Polym. Degrad. Stab. 2010, 95, 1928–1933. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, J.; Huang, S.; Deng, H. Effects of α-ZrP on Crystallinity and Flame-Retardant Behaviors of PA6/MCA Composites. Int. J. Polym. Sci. 2017, 2017, 6034741. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Li, W.; Wang, X.; Zhang, X.; Cheng, Z. Effect of Different Ionic Layered Compounds Decorated with Zinc Hydroxystannate on Flame Retardancy and Smoke Performance of Epoxy Resin. Polym. Adv. Technol. 2019, 31, 731–740. [Google Scholar] [CrossRef]
- Laipan, M.; Xiang, L.; Yu, J.; Martin, B.R.; Zhu, R.; Zhu, J.; He, H.; Clearfield, A.; Sun, L. Layered Intercalation Compounds: Mechanisms, New Methodologies, and Advanced Applications. Prog. Mater. Sci. 2020, 109, 100631. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, S. Zirconium Phosphate (ZrP)-Based Functional Materials: Synthesis, Properties and Applications. Mater. Des. 2018, 155, 19–35. [Google Scholar] [CrossRef]
- Alberti, G.; Torracca, E. Crystalline Insoluble Salts of Polybasic Metals—II. Synthesis of Crystalline Zirconium or Titanium Phosphate by Direct Precipitation. J. Inorg. Nucl. Chem. 1968, 30, 317–318. [Google Scholar] [CrossRef]
- Shuai, M.; Mejia, A.F.; Chang, Y.-W.; Cheng, Z. Hydrothermal Synthesis of Layered α-Zirconium Phosphate Disks: Control of Aspect Ratio and Polydispersity for Nano-Architecture. CrystEngComm 2013, 15, 1970. [Google Scholar] [CrossRef]
- Sun, L.; Boo, W.J.; Sue, H.-J.; Clearfield, A. Preparation of α-Zirconium Phosphate Nanoplatelets with Wide Variations in Aspect Ratios. New J. Chem. 2007, 31, 39–43. [Google Scholar] [CrossRef]
- Contreras-Ramirez, A.; Tao, S.; Day, G.S.; Bakhmutov, V.I.; Billinge, S.J.L.; Zhou, H.-C. Zirconium Phosphate: The Pathway from Turbostratic Disorder to Crystallinity. Inorg. Chem. 2019, 58, 14260–14274. [Google Scholar] [CrossRef]
- Horsley, S.E.; Nowell, D.V. The Preparation and Characterisation of Crystalline α-Zirconium Phosphate. J. Appl. Chem. Biotechnol. 1973, 23, 215–224. [Google Scholar] [CrossRef]
- Capitani, D.; Casciola, M.; Donnadio, A.; Vivani, R. High Yield Precipitation of Crystalline α-Zirconium Phosphate from Oxalic Acid Solutions. Inorg. Chem. 2010, 49, 9409–9415. [Google Scholar] [CrossRef]
- Tahara, S.; Takakura, Y.; Sugahara, Y. Preparation of α-Zirconium Phosphate from Fluorozirconate and Phosphoric Acid by Liquid-Phase Deposition. Chem. Lett. 2012, 41, 555–557. [Google Scholar] [CrossRef]
- Pica, M.; Donnadio, A.; Capitani, D.; Vivani, R.; Troni, E.; Casciola, M. Advances in the Chemistry of Nanosized Zirconium Phosphates: A New Mild and Quick Route to the Synthesis of Nanocrystals. Inorg. Chem. 2011, 50, 11623–11630. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, X.T.; Jaenicke, S.; Chuah, G.-K. Minimalistic Liquid-Assisted Route to Highly Crystalline α-Zirconium Phosphate. ChemSusChem 2017, 10, 3235–3242. [Google Scholar] [CrossRef]
- Tao, H.; Zhang, Y.; Gao, Y.; Sun, Z.; Yan, C.; Texter, J. Scalable Exfoliation and Dispersion of Two-Dimensional Materials—An Update. Phys. Chem. Chem. Phys. 2017, 19, 921–960. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Luo, Y.; Liu, B.; Cheng, H.-M. Preparation of 2D Material Dispersions and Their Applications. Chem. Soc. Rev. 2018, 47, 6224–6266. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, W.-H.; Yang, R.; Zhou, T.; Hou, D.; Zheng, X.; Liu, J.-H.; Huang, X.-J. Electrochemical and Density Functional Theory Investigation on High Selectivity and Sensitivity of Exfoliated Nano-Zirconium Phosphate toward Lead(II). Anal. Chem. 2013, 85, 3984–3990. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Boo, W.J.; Sun, D.; Clearfield, A.; Sue, H.-J. Preparation of Exfoliated Epoxy/α-Zirconium Phosphate Nanocomposites Containing High Aspect Ratio Nanoplatelets. Chem. Mater. 2007, 19, 1749–1754. [Google Scholar] [CrossRef]
- Aoyama, Y.; Fujimura, T.; Sasai, R. Preparation of α-Zirconium Phosphate Nanosheet Stacked Solid Films with High Transparency and Intercalating with Cationic Free-Base Porphyrins. Chem. Lett. 2019, 48, 40–42. [Google Scholar] [CrossRef]
- Casciola, M.; Alberti, G.; Donnadio, A.; Pica, M.; Marmottini, F.; Bottino, A.; Piaggio, P. Gels of Zirconium Phosphate in Organic Solvents and Their Use for the Preparation of Polymeric Nanocomposites. J. Mater. Chem. 2005, 15, 4262–4267. [Google Scholar] [CrossRef]
- Ramos-Garcés, M.V.; Sanchez, J.; Del Toro-Pedrosa, D.E.; Alvarez, I.B.; Wu, Y.; Valle, E.; Villagrán, D.; Jaramillo, T.F.; Colón, J.L. Transition Metal-Modified Exfoliated Zirconium Phosphate as an Electrocatalyst for the Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2019, 2, 3561–3567. [Google Scholar] [CrossRef]
- Huang, H.; Li, M.; Tian, Y.; Xie, Y.; Sheng, X.; Jiang, X.; Zhang, X. Exfoliation and Functionalization of α-Zirconium Phosphate in One Pot for Waterborne Epoxy Coatings with Enhanced Anticorrosion Performance. Prog. Org. Coat. 2020, 138, 105390. [Google Scholar] [CrossRef]
- Zhou, Y.; Ding, H.; Liu, J.; LaChance, A.M.; Xiao, M.; Meng, Y.; Sun, L. Gold Nanoparticles Immobilized on Single-Layer α-Zirconium Phosphate Nanosheets as a Highly Effective Heterogeneous Catalyst. Adv. Compos. Hybrid Mater. 2019, 2, 520–529. [Google Scholar] [CrossRef]
- Campoccia, D.; Ravaioli, S.; Vivani, R.; Donnadio, A.; Vischini, E.; Russo, A.; Visai, L.; Arciola, C.R.; Montanaro, L.; Nocchetti, M. Antibacterial Properties of a Novel Zirconium Phosphate-Glycinediphosphonate Loaded with Either Zinc or Silver. Materials 2019, 12, 3184. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-N.; Keller, S.W.; Mallouk, T.E.; Schmitt, J.; Decher, G. Characterization of Zirconium Phosphate/Polycation Thin Films Grown by Sequential Adsorption Reactions. Chem. Mater. 1997, 9, 1414–1421. [Google Scholar] [CrossRef]
- Kaschak, D.M.; Johnson, S.A.; Hooks, D.E.; Kim, H.-N.; Ward, M.D.; Mallouk, T.E. Chemistry on the Edge: A Microscopic Analysis of the Intercalation, Exfoliation, Edge Functionalization, and Monolayer Surface Tiling Reactions of α-Zirconium Phosphate. J. Am. Chem. Soc. 1998, 120, 10887–10894. [Google Scholar] [CrossRef]
- Chen, L.; Sun, D.; Li, J.; Zhu, G. Exfoliation of Layered Zirconium Phosphate Nanoplatelets by Melt Compounding. Mater. Des. 2017, 122, 247–254. [Google Scholar] [CrossRef]
- Xia, F.; Yong, H.; Han, X.; Sun, D. Small Molecule-Assisted Exfoliation of Layered Zirconium Phosphate Nanoplatelets by Ionic Liquids. Nanoscale Res. Lett. 2016, 11, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Schmelter, D.; Imanian, S.; Hintze-Bruening, H. Hierarchically Ordered α-Zirconium Phosphate Platelets in Aqueous Phase with Empty Liquid. Sci. Rep. 2019, 9, 16389. [Google Scholar] [CrossRef] [Green Version]
- White, K.L.; Li, P.; Yao, H.; Nishimura, R.; Sue, H.-J. Effect of Surface Modifier on Flow Properties of Epoxy Suspensions Containing Model Plate-like Nanoparticles. Rheol. Acta 2014, 53, 571–583. [Google Scholar] [CrossRef]
- Pica, M.; Vivani, R.; Donnadio, A.; Troni, E.; Fop, S.; Casciola, M. Small Is Beautiful: The Unusual Transformation of Nanocrystalline Layered α-Zirconium Phosphate into a New 3D Structure. Inorg. Chem. 2015, 54, 9146–9153. [Google Scholar] [CrossRef]
- Hashimoto, C.; Nakajima, Y.; Terada, T.; Itoh, K.; Nakayama, S. Effect of the Preparation Conditions of Zirconium Phosphate on the Characteristics of Sr Immobilization. J. Nucl. Mater. 2011, 408, 231–235. [Google Scholar] [CrossRef]
- Zhang, F.; Xie, Y.; Lu, W.; Wang, X.; Xu, S.; Lei, X. Preparation of Microspherical α-Zirconium Phosphate Catalysts for Conversion of Fatty Acid Methyl Esters to Monoethanolamides. J. Colloid Interface Sci. 2010, 349, 571–577. [Google Scholar] [CrossRef]
- Tarafdar, A.; Panda, A.B.; Pradhan, N.C.; Pramanik, P. Synthesis of Spherical Mesostructured Zirconium Phosphate with Acidic Properties. Microporous Mesoporous Mater. 2006, 95, 360–365. [Google Scholar] [CrossRef]
- Bashir, A.; Malik, L.A.; Dar, G.N.; Pandith, A.H. Microwave-Assisted Hydrothermal Synthesis of Agglomerated Spherical Zirconium Phosphate for Removal of Cs+ and Sr2+ Ions from Aqueous System. In Applications in Ion Exchange Materials in the Environment; Ahamed, M., Asiri, A., Eds.; Springer: Basel, Switzerland, 2019; pp. 95–108. [Google Scholar] [CrossRef]
- Mu, W.; Yu, Q.; Zhang, R.; Li, X.; Hu, R.; He, Y.; Wei, H.; Jian, Y.; Yang, Y. Controlled Fabrication of Flower-like α-Zirconium Phosphate for the Efficient Removal of Radioactive Strontium from Acidic Nuclear Wastewater. J. Mater. Chem. A 2017, 5, 24388–24395. [Google Scholar] [CrossRef]
- Yu, J.; Ding, H.; Lampron, J.; Martin, B.R.; Clearfield, A.; Sun, L. Complexing Agent Directed Growth of α-Zirconium Phosphate-Based Hexagonal Prisms. Inorg. Chem. 2020, 59, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, R.; Xing, Y. Simple One-Step Synthesis of Coil-like Cobalt Zirconium Phosphate Microspheres and the Application as Photocatalysts. Mater. Lett. 2020, 264, 127299. [Google Scholar] [CrossRef]
- Feng, Y.; He, W.; Zhang, X.; Jia, X.; Zhao, H. The Preparation of Nanoparticle Zirconium Phosphate. Mater. Lett. 2007, 61, 3258–3261. [Google Scholar] [CrossRef]
- Whittingham, M.S. Intercalation Chemistry: An Introduction. In Intercalation Chemistry; Whittingham, M.S., Jacobson, A.J., Eds.; Academic Press: New York, NY, USA, 1982; pp. 1–18. [Google Scholar] [CrossRef]
- Alberti, G.; Costantino, U.; Gupta, J.P. Crystalline Insoluble Acid Salts of Tetravalent Metals—XIX: Na+-Catalyzed H+-Mg2+ and H+-Cs+ Ion Exchanges on α-Zirconium Phosphate. J. Inorg. Nucl. Chem. 1974, 36, 2109–2114. [Google Scholar] [CrossRef]
- Alberti, G.; Bertrami, R.; Costantino, U. Crystalline Insoluble Acid Salts of Tetravalent Metals—XXII: Effect of Small Amounts of Na+ on the Ion Exchange of Alkaline Earth Metal Ions on Crystalline Zr(HPO4)2·H2O. J. Inorg. Nucl. Chem. 1976, 38, 1729–1732. [Google Scholar] [CrossRef]
- Alberti, G.; Costantino, U. Recent Progress in the Intercalation Chemistry of Layered α-Zirconium Phosphate and Its Derivatives, and Future Perspectives for Their Use in Catalysis. J. Mol. Catal. 1984, 27, 235–250. [Google Scholar] [CrossRef]
- Clearfield, A.; Tindwa, R.M. On the Mechanism of Ion Exchange in Zirconium Phosphates—XXI Intercalation of Amines by α-Zirconium Phosphate. J. Inorg. Nucl. Chem. 1979, 41, 871–878. [Google Scholar] [CrossRef]
- Kijima, T. Direct Preparation of θ-Zirconium Phosphate. Bull. Chem. Soc. Jpn. 1982, 55, 3031–3032. [Google Scholar] [CrossRef]
- Alberti, G.; Costantino, U.; Gill, J.S. Crystalline Insoluble Acid Salts of Tetravalent Metals—XXIII: Preparation and Main Ion Exchange Properties of Highly Hydrated Zirconium Bis Monohydrogen Orthophosphates. J. Inorg. Nucl. Chem. 1976, 38, 1733–1738. [Google Scholar] [CrossRef]
- Sun, L.; O’Reilly, J.Y.; Kong, D.; Su, J.Y.; Boo, W.J.; Sue, H.-J.; Clearfield, A. The Effect of Guest Molecular Architecture and Host Crystallinity upon the Mechanism of the Intercalation Reaction. J. Colloid Interface Sci. 2009, 333, 503–509. [Google Scholar] [CrossRef]
- Hu, H.; Martin, J.C.; Zhang, M.; Southworth, C.S.; Xiao, M.; Meng, Y.; Sun, L. Immobilization of Ionic Liquids in θ-Zirconium Phosphate for Catalyzing the Coupling of CO2 and Epoxides. RSC Adv. 2012, 2, 3810–3815. [Google Scholar] [CrossRef]
- Wen, M.; Mori, K.; Kuwahara, Y.; Yamashita, H. Visible-Light-Responsive Carbon Dioxide Reduction System: Rhenium Complex Intercalated into a Zirconium Phosphate Layered Matrix. ChemCatChem 2015, 7, 3519–3525. [Google Scholar] [CrossRef]
- Rivera, E.J.; Barbosa, C.; Torres, R.; Rivera, H.; Fachini, E.R.; Green, T.W.; Connick, W.B.; Colón, J.L. Luminescence Rigidochromism and Redox Chemistry of Pyrazolate-Bridged Binuclear Platinum(II) Diimine Complex Intercalated into Zirconium Phosphate Layers. Inorg. Chem. 2012, 51, 2777–2784. [Google Scholar] [CrossRef]
- Rivera, E.J.; Barbosa, C.; Torres, R.; Grove, L.; Taylor, S.; Connick, W.B.; Clearfield, A.; Colón, J.L. Vapochromic and Vapoluminescent Response of Materials Based on Platinum(II) Complexes Intercalated into Layered Zirconium Phosphate. J. Mater. Chem. 2011, 21, 15899–15902. [Google Scholar] [CrossRef]
- Saxena, V.; Diaz, A.; Clearfield, A.; Batteas, J.D.; Hussain, M.D. Zirconium Phosphate Nanoplatelets: A Biocompatible Nanomaterial for Drug Delivery to Cancer. Nanoscale 2013, 5, 2328–2336. [Google Scholar] [CrossRef]
- González, M.L.; Ortiz, M.; Hernández, C.; Cabán, J.; Rodríguez, A.; Colón, J.L.; Báez, A. Zirconium Phosphate Nanoplatelet Potential for Anticancer Drug Delivery Applications. J. Nanosci. Nanotechnol. 2016, 16, 117–129. [Google Scholar] [CrossRef]
- Song, F.; Bai, L.; Moysiadou, A.; Lee, S.; Hu, C.; Liardet, L.; Hu, X. Transition Metal Oxides as Electrocatalysts for the Oxygen Evolution Reaction in Alkaline Solutions: An Application-Inspired Renaissance. J. Am. Chem. Soc. 2018, 140, 7748–7759. [Google Scholar] [CrossRef]
- Montoya, J.H.; Seitz, L.C.; Chakthranont, P.; Vojvodic, A.; Jaramillo, T.F.; Nørskov, J.K. Materials for Solar Fuels and Chemicals. Nat. Mater. 2017, 16, 70–81. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of Electrocatalysts for Oxygen- and Hydrogen-Involving Energy Conversion Reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking Hydrogen Evolving Reaction and Oxygen Evolving Reaction Electrocatalysts for Solar Water Splitting Devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.; McCrory, C.C.L.; Ferrer, I.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking Nanoparticulate Metal Oxide Electrocatalysts for the Alkaline Water Oxidation Reaction. J. Mater. Chem. A 2016, 4, 3068–3076. [Google Scholar] [CrossRef] [Green Version]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining Theory and Experiment in Electrocatalysis: Insights into Materials Design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, J.; Ramos-Garcés, M.V.; Narkeviciute, I.; Colón, J.L.; Jaramillo, T.F. Transition Metal-Modified Zirconium Phosphate Electrocatalysts for the Oxygen Evolution Reaction. Catalysts 2017, 7, 132. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Garcés, M.V.; Sanchez, J.; Barraza Alvarez, I.; Wu, Y.; Villagrán, D.; Jaramillo, T.F.; Colón, J.L. Water Splitting Electrocatalysis within Layered Inorganic Nanomaterials. In Water Chemistry; Eyvaz, M., Yüksel, E., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Garcés, M.V.; Sanchez, J.; Luz-Rivera, K.L.; Toro-Pedrosa, D.E.D.; Jaramillo, T.F.; Colón, J.L. Morphology Control of Metal-Modified Zirconium Phosphate Support Structures for the Oxygen Evolution Reaction. Dalton Trans. 2020. [Google Scholar] [CrossRef]
- Clearfield, A. Inorganic Ion Exchangers with Layered Structures. Annu. Rev. Mater. Sci. 1984, 14, 205–229. [Google Scholar] [CrossRef]
- Xu, D.; Stevens, M.; Cosby, M.; Oener, S.Z.; Smith, A.; Enman, L.J.; Ayers, K.E.; Capuano, C.B.; Renner, J.; Danilovic, N.; et al. Earth-Abundant Oxygen Electrocatalysts for Alkaline Anion-Exchange-Membrane Water Electrolysis: Effects of Catalyst Conductivity and Comparison with Performance in Three-Electrode Cells. ACS Catal. 2019, 9, 7–15. [Google Scholar] [CrossRef]











| Method | Morphology | Size (nm) | Reference |
|---|---|---|---|
| Reflux | Hexagonal platelets | 60–200 | [34] |
| HF | Hexagonal platelets | 2000–4000 | [34] |
| Hydrothermal | Hexagonal platelets | 400–1200 | [34] |
| Oxalic acid precipitation | Hexagonal platelets | 2000–3000 | [37] |
| Liquid-phase deposition | Hexagonal platelets | 2000–3000 | [38] |
| Alcohol intercalation/deintercalation (60 °C) | Hexagonal platelets | 30–200 | [39] |
| Minimal solvent | Hexagonal platelets | 100–500 | [40] |
| Minimal solvent (with F−) | Rod-like | 4000–10,000 | [40] |
| Alcohol intercalation/deintercalation (120 °C) | Cube-like | 100–500 | [57] |
| Colloid mill procedure | Spheres | 5000–445,000 | [59] |
| Tarafdar et al. | Spheres | 1000–3000 | [60] |
| Microwave-assisted hydrothermal | Spheres | 1000–3000 | [61] |
| Mu et al. | Flower-like | 5000–7000 | [62] |
| Yu et al. | Hexagonal prisms | 1000–3000 | [63] |
| Metal | ZrP Support | Mass Activity at 1.58 V vs. RHE (A/g) | Reference |
|---|---|---|---|
| Co | α-ZrP | 115 | [87] |
| ZrP rods | 4 | [87] | |
| ZrP cubes | 72 | [87] | |
| ZrP spheres | 56 | [87] | |
| Ni | α-ZrP | 50 | [87] |
| ZrP rods | 85 | [87] | |
| ZrP cubes | 91 | [87] | |
| ZrP spheres | 272 | [87] | |
| IrOx | - | 257 | [91] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Garcés, M.V.; Colón, J.L. Preparation of Zirconium Phosphate Nanomaterials and Their Applications as Inorganic Supports for the Oxygen Evolution Reaction. Nanomaterials 2020, 10, 822. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10050822
Ramos-Garcés MV, Colón JL. Preparation of Zirconium Phosphate Nanomaterials and Their Applications as Inorganic Supports for the Oxygen Evolution Reaction. Nanomaterials. 2020; 10(5):822. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10050822
Chicago/Turabian StyleRamos-Garcés, Mario V., and Jorge L. Colón. 2020. "Preparation of Zirconium Phosphate Nanomaterials and Their Applications as Inorganic Supports for the Oxygen Evolution Reaction" Nanomaterials 10, no. 5: 822. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10050822