Antibacterial and Immunomodulatory Potentials of Biosynthesized Ag, Au, Ag-Au Bimetallic Alloy Nanoparticles Using the Asparagus racemosus Root Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Botanical Material
2.2. Preparation of Root Biomass
2.3. Preparation of Green Synthesis Ag, Au, and Ag-Au Bimetallic Nanoparticles
2.4. Characterization of Green Synthesis Ag-Au Bimetallic Nanoparticles
2.5. Biomedical Studies for the Ag, Au, and Ag-Au Bimetallic Alloy Nanoparticles
2.5.1. Antibacterial Activities
2.5.2. MIC and MBC Determination of Biosynthesized Nanoparticles
2.5.3. Morphological Study of S. aureus and P. aeuroginosa
2.5.4. Immunomodulation Activity
3. Results and Discussion
3.1. Structural Characterization of Nanoparticles
3.2. Antibacterial Activity of Ag, Au, Ag-Au Alloy Nanoparticles
3.3. MIC and MBC of Biosythesized Nanoparticles Against S. aureus and P. aeruginosa
3.4. Morphological study of S. aureus and P. aeruginosa (SEM)
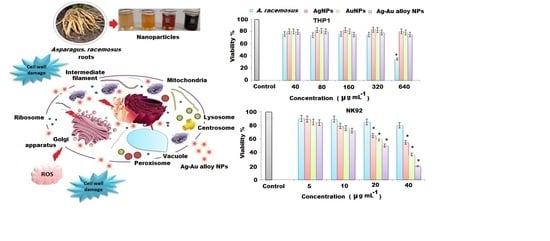
3.5. Immunomodulation Activity of Ag, Au, Ag-Au Bimetallic Alloy Nanoparticles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Rao, C.R.; Kulkarni, G.U.; Thomas, P.J.; Edwards, P.P. Metal nanoparticles and their assemblies. Chem. Soc. Rev. 2000, 29, 27–35. [Google Scholar] [CrossRef]
- MubarakAli, D.; Thajuddin, N.; Jeganathan, K.; Gunasekaran, M. Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf. B 2011, 85, 360–365. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Yamal, G.; Sharmila, P.; Rao, K.S.; Pardha-Saradhi, P. Yeast Extract Mannitol medium and its constituents promote synthesis of Au nanoparticles. Process Biochem. 2013, 48, 532–538. [Google Scholar] [CrossRef]
- Rangayasami, A.; Kannan, K.; Joshi, S.; Subban, M. Bioengineered silver nanoparticles using Elytraria acaulis (Lf) Lindau leaf extract and its biological applications. Biocatal. Agric. Biotechnol. 2020, 27, 101690. [Google Scholar] [CrossRef]
- Wijnhoven, S.W.; Peijnenburg, W.J.; Herberts, C.A.; Hagens, W.I.; Oomen, A.G.; Heugens, E.H.; Roszek, B.; Bisschops, J.; Gosens, I.; Van De Meent, D.; et al. Nano-silver—A review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 2009, 3, 109–138. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Pollini, M.; Paladini, F.; Catalano, M.; Taurino, A.; Licciulli, A.; Maffezzoli, A.; Sannino, A. Antibacterial coatings on haemodialysis catheters by photochemical deposition of silver nanoparticles. J. Mater. Sci. Mater. Med. 2011, 22, 2005–2012. [Google Scholar] [CrossRef]
- Patil, R.S.; Kokate, M.R.; Kolekar, S.S. Bioinspired synthesis of highly stabilized silver nanoparticles using Ocimum tenuiflorum leaf extract and their antibacterial activity. Spectrochim. Acta A 2012, 91, 234–238. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Mukherjee, P. Biological properties of “naked” metal nanoparticles. Adv. Drug Deliv. Rev. 2008, 60, 1289–1306. [Google Scholar] [CrossRef]
- Torres-Chavolla, E.; Ranasinghe, R.J.; Alocilja, E.C. Characterization and functionalization of biogenic gold nanoparticles for biosensing enhancement. IEEE Trans. Nanotechnol. 2010, 9, 533–538. [Google Scholar] [CrossRef]
- Rajeshkumar, S. Anticancer activity of eco-friendly gold nanoparticles against lung and liver cancer cells. J. Genet. Eng. Biotechnol. 2016, 14, 195–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.; Yang, X.; Gao, G.; Wang, J.; Lu, H.; Liu, J.; Tong, M.; Liang, X. Selective oxidation of methanol to methyl formate on catalysts of Au–Ag alloy nanoparticles supported on titania under UV irradiation. Green Chem. 2014, 16, 3603–3615. [Google Scholar] [CrossRef]
- Ramamurthy, C.H.; Padma, M.; Mareeswaran, R.; Suyavaran, A.; Kumar, M.S.; Premkumar, K.; Thirunavukkarasu, C. The extracellular synthesis of gold and silver nanoparticles and their free radical scavenging and antibacterial properties. Colloids Surf. B 2013, 102, 808–815. [Google Scholar] [CrossRef]
- Hulla, J.E.; Sahu, S.C.; Hayes, A.W. Nanotechnology: History and future. Hum. Exp. Toxicol. 2015, 34, 1318–1321. [Google Scholar] [CrossRef]
- Gupta, P.; Mahajan, A. Green chemistry approaches as sustainable alternatives to conventional strategies in the pharmaceutical industry. RSC Adv. 2015, 5, 26686–26705. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Huang, Y.; Parsons, J.G.; Zhao, L.; Lopez-Moreno, L.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L. Plant-based green synthesis of metallic nanoparticles: Scientific curiosity or a realistic alternative to chemical synthesis? Nanotechnol. Environ. Eng. 2016, 1, 4. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An overview. Biol. Trace Elem. Res. 2020. [Google Scholar] [CrossRef]
- Awwad, A.M.; Amer, M.W.; Salem, N.M.; Abdeen, A.O. Green synthesis of zinc oxide nanoparticles (ZnO-NPs) using Ailanthus altissima fruit extracts and antibacterial activity. Chem. Int. 2020, 6, 151–159. [Google Scholar] [CrossRef]
- Akter, S.; Huq, M.A. Biologically rapid synthesis of silver nanoparticles by Sphingobium sp. MAH-11T and their antibacterial activity and mechanisms investigation against drug-resistant pathogenic microbes. Artif. Cells Nanomed. Biotechnol. 2020, 48, 672–682. [Google Scholar] [CrossRef] [Green Version]
- Ranjani, S.; Ahmed, S.M.; Adnan, M.; Kumar, S.N.; Ruckmani, K.; Hemalatha, S. Synthesis, characterization and applications of endophytic fungal nanoparticles. Inorg. Nano Met. Chem. 2020, 51, 280–287. [Google Scholar] [CrossRef]
- Zhu, H.-W.; Ge, J.; Zhao, H.-Y.; Shi, L.-A.; Huang, J.; Xu, L.; Yu, S.-H. Sponge-templating synthesis of sandwich-like reduced graphene oxide nanoplates with confined gold nanoparticles and their enhanced stability for solar evaporation. Sci. China Mater. 2020, 63, 1957–1965. [Google Scholar] [CrossRef]
- Satyanarayana, T.; Reddy, S.S. A Review on Chemical and Physical Synthesis Methods of Nanomaterials. Int. J. Res. Appl. Sci. Eng. Technol. 2018, 6, 2885–2889. [Google Scholar] [CrossRef]
- Nithya, P.; Sundrarajan, M. Ionic liquid functionalized biogenic synthesis of AgAu bimetal doped CeO2 nanoparticles from Justicia adhatoda for pharmaceutical applications: Antibacterial and anti-cancer activities. J. Photochem. Photobiol. B 2020, 202, 111706. [Google Scholar] [CrossRef] [PubMed]
- Godipurge, S.S.; Yallappa, S.; Biradar, N.J.; Biradar, J.S.; Dhananjaya, B.L.; Hegde, G.; Jagadish, K.; Hegde, G. A facile and green strategy for the synthesis of Au, Ag and Au-Ag alloy nanoparticles using aerial parts of R. hypocrateriformis extract and their biological evaluation. Enzyme Microb. Technol. 2016, 95, 74–184. [Google Scholar] [CrossRef] [PubMed]
- Chavez, K.; Rosas, G. Green Synthesis and Characterization of Ag@ Au Core-shell Bimetallic Nanoparticles using the Extract of Hamelia patens Plant. Microsc. Microanal. 2019, 25, 1102–1103. [Google Scholar] [CrossRef] [Green Version]
- Khanra, K.; Panja, S.; Choudhuri, I.; Chakraborty, A.; Bhattacharyya, N. Bactericidal and cytotoxic properties of silver nanoparticle synthesized from root extract of Asparagus racemosus. Nano Biomed. Eng. 2016, 8, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Pallela, P.N.V.K.; Ummey, S.; Ruddaraju, L.K.; Kollu, P.; Khan, S.; Pammi, S.V.N. Antibacterial activity assessment and characterization of green synthesized CuO nanorods using Asparagus racemosus roots extract. SN Appl. Sci. 2019, 1, 421. [Google Scholar] [CrossRef] [Green Version]
- Raut, R.W.; Haroon, A.S.M.; Malghe, Y.S.; Nikam, B.T.; Kashid, S.B. Rapid biosynthesis of platinum and palladium metal nanoparticles using root extract of Asparagus racemosus Linn. Adv. Mater. Lett. 2013, 4, 650–654. [Google Scholar] [CrossRef]
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles alter secondary metabolism in plants via ROS burst. Front. Plant Sci. 2017, 8, 832. [Google Scholar] [CrossRef] [Green Version]
- Hasan, N.; Ahmad, N.; Zohrameena, S.; Khalid, M.; Akhtar, J. Asparagus racemosus: For medicinal uses & pharmacological actions. Int. J. Adv. Res. 2016, 4, 259–267. [Google Scholar]
- Mishra, J.N.; Verma, N.K. Asparagus racemosus: Chemical constituents and pharmacological activities—A review. Eur. J. Biomed. Pharm. Sci. 2017, 4, 207–213. [Google Scholar]
- Gautam, M.; Saha, S.; Bani, S.; Kaul, A.; Mishra, S.; Patil, D.; Satti, N.K.; Suri, K.A.; Gairola, S.; Suresh, K.; et al. Immunomodulatory activity of Asparagus racemosus on systemic Th1/Th2 immunity: Implications for immunoadjuvant potential. J. Ethnopharmacol. 2009, 121, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer:from tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amarante-Mendes, G.P.; Adjemian, S.; Branco, L.M.; Zanetti, L.C.; Weinlich, R.; Bortoluci, K.R. Pattern recognition receptors and the host cell death, molecular machinery. Front. Immunol. 2018, 9, 2379. [Google Scholar] [CrossRef] [Green Version]
- Blazar, B.R.; MacDonald, K.; Hill, G.R. Immune regulatory cell infusion for graft-versus-host disease prevention and therapy. Blood 2018, 131, 2651–2660. [Google Scholar] [CrossRef]
- Nocentini, G.; Migliorati, G.; Riccardi, C. The molecular and cellular mechanisms responsible for the anti-inflammatory and immunosuppressive effects of glucocorticoids. In Systemic Corticosteroids for Inflammatory Disorders in Pediatrics; Adis: Cham, Switzerland, 2015; pp. 25–41. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [Green Version]
- Meirow, Y.; Baniyash, M. Immune biomarkers for chronic inflammation related complications in non-cancerous and cancerous diseases. Cancer Immunol. Immunother. 2017, 66, 1089–1101. [Google Scholar] [CrossRef]
- Pahwa, R.; Jialal, I. Chronic Inflammation; Updated 24 March 2018; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Sheth, A.N. Can Anti-Inflammatory Drugs Fight Infection? Sci. Transl. Med. 2013, 5, 192ec110. [Google Scholar] [CrossRef]
- Petrarca, C.; Clemente, E.; Amato, V.; Pedata, P.; Sabbioni, E.; Bernardini, G.; Iavicoli, I.; Cortese, S.; Niu, Q.; Otsuki, T.; et al. Engineered metal based nanoparticles and innate immunity. Clin. Mol. Allergy. 2015, 13, 13. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.A.; Abdelhalim, M.A.K.; Alhomida, A.S.; Al-Ayed, M.S. Effects of naked gold nanoparticles on proinflammatory cytokines mRNA expression in rat liver and kidney. BioMed. Res. Int. 2013, 2013, 590730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumbayev, V.V.; Yasinska, I.M.; Garcia, C.P.; Gilliland, D.; Lall, G.S.; Gibbs, B.F.; Bonsall, D.R.; Varani, L.; Rossi, F.; Calzolai, L. Gold nanoparticles downregulate interleukin-1β-induced pro-inflammatory responses. Small 2013, 9, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Lateef, A.; Adelere, I.A.; Gueguim-Kana, E.B.; Asafa, T.B.; Beukes, L.S. Green synthesis of silver nanoparticles using keratinase obtained from a strain of Bacillus safensis LAU 13. Int. Nano Lett. 2015, 5, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Lateef, A.; Ojo, S.A.; Folarin, B.I.; Gueguim-Kana, E.B.; Beukes, L.S. Kolanut (Cola nitida) mediated synthesis of silver–gold alloy nanoparticles: Antifungal, catalytic, larvicidal and thrombolytic applications. J. Cluster Sci. 2016, 27, 1561–1577. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Singh, S.; Datta, S.; Kumar, S.; Bhadrecha, P.; Dhanjal, D.S.; Singh, J. Biotechnological Aspects of Nanoparticles Driven from Natural Products for Drug Delivery System and Other Applications. In Bioactive Natural Products in Drug Discovery; Springer: Singapore, 2020; pp. 549–583. [Google Scholar] [CrossRef]
- Elbagory, A.M.; Meyer, M.; Cupido, C.N.; Hussein, A.A. Inhibition of bacteria associated with wound infection by biocompatible green synthesized gold nanoparticles from South African plant extracts. Nanomaterials 2017, 7, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, K.B.; Raman, T.; Anbazhagan, V. Platinum nanoparticles inhibit bacteria proliferation and rescue zebrafish from bacterial infection. RSC Adv. 2016, 6, 44415–44424. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef]
- Kumari, M.M.; Jacob, J.; Philip, D. Green synthesis and applications of Au-Ag bimetallic nanoparticles. Spectrochim. Acta A 2015, 137, 185–192. [Google Scholar] [CrossRef]
- Zhang, G.; Du, M.; Li, Q.; Li, X.; Huang, J.; Jiang, X.; Sun, D. Green synthesis of Au–Ag alloy nanoparticles using Cacumen platycladi extract. RSC Adv. 2013, 3, 1878–1884. [Google Scholar] [CrossRef]
- Khatami, M.; Pourseyedi, S.; Khatami, M.; Hamidi, H.; Zaeifi, M.; Soltani, L. Synthesis of silver nanoparticles using seed exudates of Sinapis arvensis as a novel bioresource, and evaluation of their antifungal activity. Bioresour. Bioprocess. 2015, 2, 19. [Google Scholar] [CrossRef] [Green Version]
- Fleury, B.; Cortes-Huerto, R.; Tache, O.; Testard, F.; Menguy, N.; Spalla, O. Gold nanoparticle internal structure and symmetry probed by unified small-angle X-ray scattering and X-ray diffraction coupled with molecular dynamics analysis. Nano Lett. 2015, 15, 6088–6094. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Srivastava, A.; Kumar, V.; Singh, K. Phytochemicals, medicinal and food applications of Shatavari (Asparagus racemosus): An updated review. Nat. Prod. J. 2018, 8, 32–44. [Google Scholar] [CrossRef]
- Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Kilmartin, P.A.; Versari, A. Application of Fourier transform infrared (FTIR) spectroscopy in the characterization of tannins. Appl. Spectrosc. Rev. 2015, 50, 407–442. [Google Scholar] [CrossRef]
- Elemike, E.E.; Onwudiwe, D.C.; Fayemi, O.E.; Botha, T.L. Green synthesis and electrochemistry of Ag, Au, and Ag–Au bimetallic nanoparticles using goldenrod (Solidago canadensis) leaf extract. Appl. Phys. A 2019, 125, 42. [Google Scholar] [CrossRef]
- Narchin, F.; Larijani, K.; Rustaiyan, A.; Ebrahimi, S.N.; Tafvizi, F. Phytochemical synthesis of silver nanoparticles by two techniques Using Saturaja rechengri Jamzad extract: Identifying and comparing in Vitro anti-proliferative activities. Adv. Pharm. Bull. 2018, 8, 235. [Google Scholar] [CrossRef]
- Zahin, N.; Anwar, R.; Tewari, D.; Kabir, M.T.; Sajid, A.; Mathew, B.; Uddin, M.S.; Aleya, L.; Abdel-Daim, M.M. Nanoparticles and its biomedical applications in health and diseases: Special focus on drug delivery. Environ. Sci. Pollut. R. 2010, 27, 19151–19168. [Google Scholar] [CrossRef]
- McNamara, K.; Tofail, S.A. Nanosystems: The use of nanoalloys, metallic, bimetallic, and magnetic nanoparticles in biomedical applications. Phys. Chem. Chem. Phys. 2015, 17, 27981–27995. [Google Scholar] [CrossRef]
- Azzam, E.M.S.; Zaki, M.F. Surface and antibacterial activity of synthesized nonionic surfactant assembled on metal nanoparticles. Egypt. J. Pet. 2016, 25, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Jena, P.; Bhattacharya, M.; Bhattacharjee, G.; Satpati, B.; Mukherjee, P.; Senapati, D.; Srinivasan, R. Bimetallic gold–silver nanoparticles mediate bacterial killing by disrupting the actin cytoskeleton MreB. Nanoscale 2020, 12, 3731–3749. [Google Scholar] [CrossRef]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941. [Google Scholar] [CrossRef] [Green Version]
- Pandiyan, N.; Murugesan, B.; Arumugam, M.; Sonamuthu, J.; Samayanan, S.; Mahalingam, S. Ionic liquid-A greener templating agent with Justicia adhatoda plant extract assisted green synthesis of morphologically improved Ag-Au/ZnO nanostructure and it’s antibacterial and anticancer activities. J. Photochem. Photobiol. B Biol. 2019, 198, 111559. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javaid, A.; Oloketuyi, S.F.; Khan, M.M.; Khan, F. Diversity of bacterial synthesis of silver nanoparticles. BioNanoScience 2018, 8, 43–59. [Google Scholar] [CrossRef]
- Luo, Y.H.; Chang, L.W.; Lin, P. Metal-based nanoparticles and the immune system: Activation, inflammation, and potential applications. Biomed. Res. Int. 2015, 2015, 143720. [Google Scholar] [CrossRef]
- Weng, Y.; Li, J.; Ding, X.; Wang, B.; Dai, S.; Zhou, Y.; Pang, R.; Zhao, Y.; Xu, H.; Tian, B.; et al. Functionalized Gold and Silver Bimetallic Nanoparticles Using Deinococcus radiodurans Protein Extract Mediate Degradation of Toxic Dye Malachite Green. Int. J. Nanomed. 2020, 15, 1823. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.H.; Jeong, S.; Choi, H.J.; Eun, H.; Jo, M.G.; Kwon, W.Y.; Kim, S.; Kim, Y.; Lee, M.; Park, K.S. Target-induced aggregation of gold nanoparticles for colorimetric detection of Bisphenol A. J. Nanomater. 2019, 2019, 3676384. [Google Scholar] [CrossRef] [Green Version]
- Lara-Reyna, S.; Holbrook, J.; Jarosz-Griffiths, H.H.; Peckham, D.; McDermott, M.F. Dysregulated signalling pathways in innate immune cells with cystic fibrosis mutations. Cell. Mol. Life Sci. 2020, 1–19. [Google Scholar] [CrossRef]
- Seow, V.; Lim, J.; Iyer, A.; Suen, J.Y.; Ariffin, J.K.; Hohenhaus, D.M.; Sweet, M.J.; Fairlie, D.P. Inflammatory responses induced by lipopolysaccharide are amplified in primary human monocytes but suppressed in macrophages by complement protein C5a. J. Immunol. 2013, 191, 4308–4316. [Google Scholar] [CrossRef] [Green Version]
- Wajant, H.; Siegmund, D. TNFR1 and TNFR2 in the control of the life and death balance of macrophages. Front. Cell Dev. Biol. 2019, 7, 91. [Google Scholar] [CrossRef]
- Cohen, P. The TLR and IL-1 signalling network at a glance. J. Cell Sci. 2014, 127, 2383–2390. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Navajas, J.M.; Lee, J.; David, M.; Raz, E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 2012, 12, 125–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gessani, S.; Belardelli, F. IFN-γ expression in macrophages and its possible biological significance. Cytokine Growth Factor Rev. 1998, 9, 117–123. [Google Scholar] [CrossRef]
- Ye, J.; Ortaido, J.R.; Conlon, K.; Winkler-Pickett, R.; Young, H.A. Cellular and molecular mechanisms of IFN-γ production induced by IL-2 and IL-12 in a human NK cell line. J. Leukocyte Biol. 1995, 58, 225–233. [Google Scholar] [CrossRef]
- Bream, J.H.; Curiel, R.E.; Yu, C.R.; Egwuagu, C.E.; Grusby, M.J.; Aune, T.M.; Young, H.A. IL-4 synergistically enhances both IL-2–and IL-12–induced IFN-γ expression in murine NK cells. Blood 2003, 102, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, P.; Campbell, B.J.; Rhodes, J.M. Bacteria in the pathogenesis of inflammatory bowel disease. Biochem. Soc. Trans. 2011, 39, 1067–1072. [Google Scholar] [CrossRef]
- Rauch, I.; Müller, M.; Decker, T. The regulation of inflammation by interferons and their STATs. JAK STAT 2013, 2, e23820. [Google Scholar] [CrossRef] [Green Version]
- Bekic, M.; Tomic, S.; Rudolf, R.; Milanovic, M.; Vucevic, D.; Anzel, I.; Colic, M. The effect of stabilisation agents on the immunomodulatory properties of gold nanoparticles obtained by ultrasonic spray pyrolysis. Materials 2019, 12, 4121. [Google Scholar] [CrossRef] [Green Version]
- Farrugia, M.; Baron, B. The role of TNF-α in rheumatoid arthritis: A focus on regulatory T cells. J. Clin. Transl. Res. 2016, 2, 84. [Google Scholar] [CrossRef]











| S.No. | Samples | Antibacterial Activity | |||
|---|---|---|---|---|---|
| S. aureus | P. aeruginosa | ||||
| Concentration (µg mL−1) | Zone of Inhibition (mm) | Concentration (µg mL−1) | Zone of Inhibition (mm) | ||
| 1 | Plant extract | 20 | 15 | 20 | 17 |
| 40 | 19 | 40 | 21 | ||
| 80 | 20 | 80 | 22 | ||
| 2 | AgNPs | 20 | 18 | 20 | 21 |
| 40 | 22 | 40 | 24 | ||
| 80 | 24 | 80 | 28 | ||
| 3 | AuNPs | 20 | 17 | 20 | 19 |
| 40 | 20 | 40 | 24 | ||
| 80 | 23 | 80 | 26 | ||
| 4 | Ag-Au alloy NPs | 20 | 22 | 20 | 25 |
| 40 | 26 | 40 | 29 | ||
| 80 | 30 | 80 | 33 | ||
| Sample | CFU mL−1 | |
|---|---|---|
| P. aeruoginosa | S. aureus | |
| Control | TNTC | TNTC |
| 5 | TNTC | TNTC |
| 15 | TNTC | TNTC |
| 30 | TNTC | TNTC |
| 60 | TNTC | TNTC |
| 120 | TNTC | TNTC |
| 240 | 5 × 104 | 3 × 102 |
| 480 | 1 × 102 | 148 |
| 960 | NIL | 5 |
| 1920 | NIL | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amina, M.; Al Musayeib, N.M.; Alarfaj, N.A.; El-Tohamy, M.F.; Al-Hamoud, G.A. Antibacterial and Immunomodulatory Potentials of Biosynthesized Ag, Au, Ag-Au Bimetallic Alloy Nanoparticles Using the Asparagus racemosus Root Extract. Nanomaterials 2020, 10, 2453. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10122453
Amina M, Al Musayeib NM, Alarfaj NA, El-Tohamy MF, Al-Hamoud GA. Antibacterial and Immunomodulatory Potentials of Biosynthesized Ag, Au, Ag-Au Bimetallic Alloy Nanoparticles Using the Asparagus racemosus Root Extract. Nanomaterials. 2020; 10(12):2453. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10122453
Chicago/Turabian StyleAmina, Musarat, Nawal M. Al Musayeib, Nawal A. Alarfaj, Maha F. El-Tohamy, and Gadah A. Al-Hamoud. 2020. "Antibacterial and Immunomodulatory Potentials of Biosynthesized Ag, Au, Ag-Au Bimetallic Alloy Nanoparticles Using the Asparagus racemosus Root Extract" Nanomaterials 10, no. 12: 2453. https://0-doi-org.brum.beds.ac.uk/10.3390/nano10122453