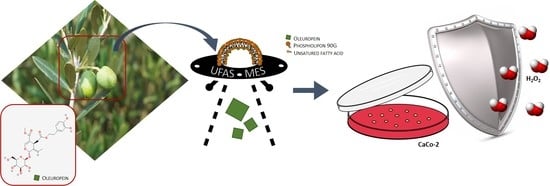
Oleuropein-Laded Ufasomes Improve the Nutraceutical Efficacy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
2.3. Physico-Chemical and Technological Characterization of Carriers
2.4. Transmission Electron Microscopy (TEM)
2.5. Entrapment Efficacy of Samples
2.6. Oleuropein Release Profiles
2.7. HPLC Analysis
2.8. Cell Cultures and In Vitro Cytotoxic and Antioxidant Evaluation
2.9. Confocal Laser Scanning Microscopy (CLSM)
2.10. Statistical Analysis
3. Results
3.1. Physico-Chemical and Technological Characterization of Vesicular Carriers
3.2. In Vitro Cytotoxic Evaluation
3.3. Ufasomes-Cell Interaction
3.4. Oleuropein-Loaded Ufasomes Preparation and Physico-Chemical Characterization
3.5. Evaluation of Oleuropein Release Profile
3.6. Antioxidant Activity Evaluation: MTT and LDH Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Felice, S.L. The Nutraceutical Revolution: Fueling a Powerful, New International Market; Harvard University Advanced Management Program in Biomedical Research and Development: Como, Italy, 1989. [Google Scholar]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef]
- Rigacci, S.; Stefani, M. Nutraceutical Properties of Olive Oil Polyphenols. An Itinerary from Cultured Cells through Animal Models to Humans. Int. J. Mol. Sci. 2016, 17, 843. [Google Scholar] [CrossRef] [Green Version]
- Rahmani, A.H.; Albutti, A.S.; Aly, S.M. Therapeutics role of olive fruits/oil in the prevention of diseases via modulation of anti-oxidant, anti-tumour and genetic activity. Int. J. Clin. Exp. Med. 2014, 7, 799–808. [Google Scholar]
- Ma, S.C.; He, Z.D.; Deng, X.L.; But, P.P.; Ooi, V.E.; Xu, H.X.; Lee, S.H.; Lee, S.F. In vitro evaluation of secoiridoid glucosides from the fruits of Ligustrum lucidum as antiviral agents. Chem. Pharm. Bull. 2001, 49, 1471–1473. [Google Scholar] [CrossRef] [Green Version]
- Eidi, A.; Eidi, M.; Darzi, R. Antidiabetic effect of Olea europaea L. In normal and diabetic rats. Phytother. Res. 2009, 23, 347–350. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Critselis, E. Mediterranean diet and longevity. Eur. J. Cancer Prev. 2004, 13, 453–456. [Google Scholar] [CrossRef] [Green Version]
- De la Puerta, R.; Guttierrez, V.R.; Hoult, J.R.S. Inhibition of leukocyte 5-lipoxygenase by phenolics from virgin olive oil. Biochem. Pharmacol. 1999, 57, 445–449. [Google Scholar] [CrossRef]
- Larussa, T.; Oliverio, M.; Suraci, E.; Greco, M.; Placida, R.; Gervasi, S.; Marasco, R.; Imeneo, M.; Paolino, D.; Tucci, L.; et al. Oleuropein Decreases Cyclooxygenase-2 and Interleukin-17 Expression and Attenuates Inflammatory Damage in Colonic Samples from Ulcerative Colitis Patients. Nutrients 2017, 9, 391. [Google Scholar] [CrossRef] [Green Version]
- Elamin, M.H.; Daghestani, M.H.; Omer, S.A.; Elobeid, M.A.; Virk, P.; Al-Olayan, E.M.; Hassan, Z.K.; Mohammed, O.B.; Aboussekhra, A. Olive oil oleuropein has anti-breast cancer properties with higher efficiency on ER-negative cells. Food Chem. Toxicol. 2013, 53, 310–316. [Google Scholar] [CrossRef]
- Hamdi, H.K.; Castellon, R. Oleuropein, a non-toxic olive iridoid, is an anti-tumor agent and cytoskeleton disruptor. Biochem. Biophys. Res. Commun. 2005, 334, 769–778. [Google Scholar] [CrossRef]
- Saija, A.; Trombetta, D.; Tomaino, A.; Lo Cascio, R.; Princi, P.; Uccella, N.; Bonina, F.; Castelli, F. ‘In vitro’ evaluation of the antioxidant activity and biomembrane interaction of the plant phenols oleuropein and hydroxytyrosol. Int. J. Pharm. 1998, 166, 123–133. [Google Scholar] [CrossRef]
- Ogun, M.; Ozcan, A.; Karaman, M.; Merhan, O.; Ozen, H.; Kukurt, A.; Karapehlivan, M. Oleuropein ameliorates arsenic induced oxidative stress in mice. J. Trace Elem. Med. Biol. 2016, 36, 1–6. [Google Scholar] [CrossRef]
- Choi, S.H.; Joo, H.B.; Lee, S.J.; Choi, H.Y.; Park, Y.H.; Baek, S.H.; Kwon, S.M. Oleuropein prevents angiotensin II-mediated: Human vascular progenitor cell depletion. Int. J. Cardiol. 2015, 181, 160–165. [Google Scholar] [CrossRef]
- Ji, S.T.; Kim, Y.J.; Jung, S.Y.; Kim, D.Y.; Kang, S.; Park, J.H.; Jang, W.B.; Ha, J.; Yun, J.; Kwon, S.M. Oleuropein attenuates hydrogen peroxide-induced autophagic cell death in human adipose-derived stem cells. Biochem. Biophys. Res. Commun. 2018, 15, 675–680. [Google Scholar] [CrossRef]
- González-Ortega, R.; Šturm, L.; Skrt, M.; Di Mattia, C.D.; Pittia, P.; Ulrih, N.P. Liposomal Encapsulation of Oleuropein and an Olive Leaf Extract: Molecular Interactions, Antioxidant Effects and Applications in Model Food Systems. Food Biophys. 2020, 1–14. [Google Scholar] [CrossRef]
- Ramírez, E.; Brenes, M.; García, P.; Medina, E.; Romero, C. Oleuropein hydrolysis in natural green olives: Importance of the endogenous enzymes. Food Chem. 2016, 206, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Bonechi, C.; Donati, A.; Tamasi, G.; Pardini, A.; Rostom, H.; Leone, G.; Lamponi, S.; Consumi, M.; Magnani, A.; Rossi, C. Chemical characterization of liposomes containing nutraceutical compounds: Tyrosol, hydroxytyrosol and oleuropein. Biophys. Chem. 2019, 246, 25–34. [Google Scholar] [CrossRef]
- Huguet-Casquero, A.; Moreno-Sastre, M.; López-Méndez, T.B.; Gainza, E.; Pedraz, J.L. Encapsulation of Oleuropein in Nanostructured Lipid Carriers: Biocompatibility and Antioxidant Efficacy in Lung Epithelial Cells. Pharmaceutics 2020, 12, 429. [Google Scholar] [CrossRef]
- Huguet-Casquero, A.; Xu, Y.; Gainza, E.; Pedraz, J.L.; Beloqui, A. Oral delivery of oleuropein-loaded lipid nanocarriers alleviates inflammation and oxidative stress in acute colitis. Int. J. Pharm. 2020, 586. [Google Scholar] [CrossRef]
- Gebicki, J.; Hicks, M. Ufasomes are Stable Particles surrounded by Unsaturated Fatty Acid Membranes. Nature 1973, 243, 232–234. [Google Scholar] [CrossRef]
- Morigaki, K.; Walde, P. Fatty acid vesicles. Colloid Interface Sci. 2007, 12, 75–80. [Google Scholar] [CrossRef]
- Fan, Y.; Fang, Y.; Ma, L. The self-crosslinked ufasome of conjugated linoleic acid: Investigation of morphology, bilayer membrane and stability. Colloid Surf. B 2014, 123, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, W.R.; Deamer, D.W. Liposomes from ionic, single-chain amphiphiles. Biochemistry 1978, 17, 3759–3768. [Google Scholar] [CrossRef] [PubMed]
- Namani, T.; Ishikawa, T.; Morigaki, K.; Walde, P. Vesicles from docosahexaenoic acid. Colloid Surf. B 2007, 15, 118–123. [Google Scholar] [CrossRef]
- Naik, P.V.; Dixit, S.G. Ufasomes as Plausible Carriers for Horizontal Gene Transfer. J. Dispers. Sci. Technol. 2008, 29, 804–808. [Google Scholar] [CrossRef]
- Keys, A. Mediterranean diet and public health: Personal reflections. Am. J. Clin. Nutr. 1995, 61, 1321S–1323S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Shimizu, K.; Kondo, R. Anti-androgenic activity of fatty acids. Chem. Biodivers. 2009, 6, 503–512. [Google Scholar] [CrossRef]
- Carrillo, C.; Cavia, M.; Alonso-Torre, S.R. Oleic acid inhibits store-operated calcium entry in human colorectal adenocarcinoma cells. Eur. J. Nutr. 2012, 51, 677–684. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Froiio, F.; Mancuso, A.; Iannone, M.; Fresta, M.; Fiorito, S.; Celia, C.; Paolino, D. In vitro and in vivo trans-epidermal water loss evaluation following topical drug delivery systems application for pharmaceutical analysis. J. Pharm. Biomed. Anal. 2020, 15, 113295. [Google Scholar] [CrossRef]
- Wisniewska, M. Influences of polyacrylic acid adsorption and temperature on the alumina suspension stability. Powder Technol. 2010, 198, 258–266. [Google Scholar] [CrossRef]
- Hanafy, N.A.; Dini, L.; Citti, C.; Cannazza, G.; Leporatti, S. Inihibition of Glycolysis by Using a Micro/Nano-Lipid Bromopyruvic Chitosan Carrier as a Promising Tool to Improve Treatment of Hepatocellular Carcinoma. Nanomaterials 2018, 8, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Francesco, M.; Primavera, R.; Fiorito, S.; Cristiano, M.C.; Taddeo, V.A.; Epifano, F.; Di Marzio, L.; Genovese, S.; Celia, C. Acronychiabaueri Analogue Derivative-Loaded Ultradeformable Vesicles: Physicochemical Characterization and Potential Applications. Planta Med. 2017, 83, 482–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fresta, M.; Mancuso, A.; Cristiano, M.C.; Urbanek, C.; Cilurzo, F.; Cosco, D.; Iannone, M.; Paolino, D. Targeting of the pilosebaceous follicle by liquid crystal nanocarriers: In vitro and in vivo effects of the entrapped minoxidil. Pharmaceutics 2020, 12, 1127. [Google Scholar] [CrossRef] [PubMed]
- Dorkoosh, F.A.; Setyaningsih, D.; Borchard, G.; Rafiee-Tehrani, M.; Verhoef, J.C.; Junginger, H.E. Effects of superporous hydrogels on paracellular drug permeability and cytotoxicity studies in Caco-2 cell monolayers. Int. J. Pharm. 2002, 241, 35–45. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Froiio, F.; Spaccapelo, R.; Mancuso, A.; Nisticò, S.P.; Udongo, B.P.; Fresta, M.; Paolino, D. Sulforaphane-Loaded Ultradeformable Vesicles as A Potential Natural Nanomedicine for the Treatment of Skin Cancer Diseases. Pharmaceutics 2020, 12, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurišić, V.; Spužić, I.; Konjević, G. A comparison of the NK cell cytotoxicity with effects of TNF-α against K-562 cells, determined by LDH release assay. Cancer Lett. 1999, 138, 67–72. [Google Scholar] [CrossRef]
- Cosco, D.; Failla, P.; Costa, N.; Pullano, S.; Fiorillo, A.; Mollace, V.; Fresta, M.; Paolino, D. Rutin-loaded chitosan microspheres: Characterization and evaluation of the anti-inflammatory activity. Carbohydr. Polym. 2016, 152, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.J.; Ladouceur, H.D.; Moeller, T.; Kirui, D.; Batt, C.A. Analysis of a laminar-flow diffusional mixer for directed self-assembly of liposomes. Biomicrofluidics 2012, 6, 044119. [Google Scholar] [CrossRef] [Green Version]
- Paolino, D.; Lucania, D.; Mardente, D.; Alhaique, F.; Fresta, M. Ethosomes, for skin delivery of ammonium glycyrrhizinate: In vitro percutaneous permeation through human skin and in vivo anti-inflammatory activity on human volunteers. J. Control. Release 2005, 106, 99–110. [Google Scholar] [CrossRef]
- Celia, C.; Trapasso, E.; Cosco, D.; Paolino, D.; Fresta, M. Turbiscan lab expert analysis of the stability of ethosomes and ultradeformable liposomes containing a bilayer fluidizing agent. Colloid Surf. B 2009, 72, 155–160. [Google Scholar] [CrossRef]
- Michał, W.; Ewa, D.; Tomasz, C. Lecithin-based wet chemical precipitation of hydroxyapatite nanoparticles. Colloid Polym. Sci. 2015, 293, 1561–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, G.B.; Chacon, E.; Chacon, P.G.; Bordeaux-Rego, P.; Duarte, A.S.; Saad, S.T.O.; Zavaglia, C.A.; Cunha, M.R. Fatty acid is a potential agent for bone tissue induction: In vitro and in vivo approach. Exp. Biol. Med. 2017, 242, 1765–1771. [Google Scholar] [CrossRef]
- Rayaprolu, S.J.; Hettiarachchy, N.S.; Chen, P.; Kannan, A.; Mauromostakos, A. Peptides derived from high oleic acid soybean meals inhibit colon, liver and lung cancer cell growth. Food Res. Int. 2013, 50, 282–288. [Google Scholar] [CrossRef]
- Mericli, F.; Becer, E.; Kabadayi, H.; Hanoglu, A.; Hanoglu, D.T.; Yavuz, D.O.; Ozek, T.; Vatansever, S. Fatty acid composition and anticancer activity in colon carcinoma cell lines of Prunus dulcis seed oil. Pharm. Biol. 2017, 55, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.H.; Aburahma, M.H. Ufasomes nano-vesicles-based lyophilized platforms for intranasal delivery of cinnarizine: Preparation, optimization, ex-vivo histopathological safety assessment and mucosal confocal imaging. Pharm. Dev. Technol. 2016, 21, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef] [PubMed]
- Attama, A.A.; Muller-Goymann, C.C. Effect of beeswax modification on the lipid matrix and solid lipid nanoparticle crystallinity. Colloid Surf. A 2008, 315, 189–195. [Google Scholar] [CrossRef]
- Peters, K.; Muller, R.H.; Craig, D.Q.M. An investigation into the distribution of lecithins in nanosuspension systems using low frequency dielectric spectroscopy. Int. J. Pharm. 1999, 184, 53–61. [Google Scholar] [CrossRef]
- Wijeratne, S.S.K.; Cuppett, S.L.; Schlegel, V. Hydrogen Peroxide Induced Oxidative Stress Damage and Antioxidant Enzyme Response in Caco-2 Human Colon Cells. J. Agric. Food Chem. 2005, 53, 8768–8774. [Google Scholar] [CrossRef]










| Formulation | Oleuropein (µM) | Lipid Composition (Molar Ratio) | ||
|---|---|---|---|---|
| Oleic Acid | Linoleic Acid | PL90G® | ||
| A | - | 1 | 1 | 0.5 |
| A10 | 10 | 1 | 1 | 0.5 |
| A100 | 100 | 1 | 1 | 0.5 |
| B | - | 1 | 1 | - |
| B10 | 10 | 1 | 1 | - |
| B100 | 100 | 1 | 1 | - |
| Sample | Lipid Composition (Molar Ratio) | Mean Size (nm) | Polidispersity Index | Zeta Potential (mV) | ||
|---|---|---|---|---|---|---|
| Oleic Acid | Linoleic Acid | PL90G | ||||
| A | 1 | 1 | 0.5 | 185 ± 2 | 0.18 ± 0.01 | −43 ± 1 |
| B | 1 | 1 | - | 280 ± 5 | 0.21 ± 0.02 | −41 ± 1 |
| Sample | Mean Size (nm) | Polidispersity Index | Zeta Potential (mV) | EE (%) |
|---|---|---|---|---|
| A | 185 ± 2 | 0.18 ± 0.01 | −43 ± 1 | - |
| A10 | 184 ± 1 | 0.21 ± 0.01 | −39 ± 2 | 85 ± 1 |
| A100 | 199 ± 1 | 0.25 ± 0.03 | −42 ± 1 | 89 ± 2 |
| B | 280 ± 5 | 0.21 ± 0.02 | −41 ± 1 | - |
| B10 | 350 ± 6 | 0.84 ± 0.06 | −38 ± 5 | 45 ± 1 |
| B100 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristiano, M.C.; Froiio, F.; Mancuso, A.; Cosco, D.; Dini, L.; Di Marzio, L.; Fresta, M.; Paolino, D. Oleuropein-Laded Ufasomes Improve the Nutraceutical Efficacy. Nanomaterials 2021, 11, 105. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11010105
Cristiano MC, Froiio F, Mancuso A, Cosco D, Dini L, Di Marzio L, Fresta M, Paolino D. Oleuropein-Laded Ufasomes Improve the Nutraceutical Efficacy. Nanomaterials. 2021; 11(1):105. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11010105
Chicago/Turabian StyleCristiano, Maria Chiara, Francesca Froiio, Antonia Mancuso, Donato Cosco, Luciana Dini, Luisa Di Marzio, Massimo Fresta, and Donatella Paolino. 2021. "Oleuropein-Laded Ufasomes Improve the Nutraceutical Efficacy" Nanomaterials 11, no. 1: 105. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11010105