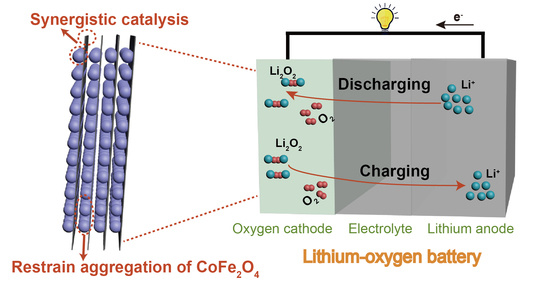
Enhancing the Capacity and Stability by CoFe2O4 Modified g-C3N4 Composite for Lithium-Oxygen Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Material Characterization
2.3. Electrochemical Performance Test
2.3.1. ORR/OER Performance Test
2.3.2. Battery Performance Test
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Zhang, Z.; Liu, W.; Zhang, Q.; Wang, X.G.; Xie, Z.; Zhou, Z. Enzyme-Inspired Room-Temperature Lithium–Oxygen Chemistry via Reversible Cleavage and Formation of Dioxygen Bonds. Angew. Chem. Int. Ed. 2020, 59, 17856–17863. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Zhou, Z. Towards practical lithium-metal anodes. Chem. Soc. Rev. 2020, 49, 3040–3071. [Google Scholar] [CrossRef]
- Qiao, Y.; He, Y.; Wu, S.; Jiang, K.; Li, X.; Guo, S.; He, P.; Zhou, H. MOF-Based Separator in an Li–O2 Battery: An Effective Strategy to Restrain the Shuttling of Dual Redox Mediators. ACS Energy Lett. 2018, 3, 463–468. [Google Scholar] [CrossRef]
- Mu, X.; Wen, Q.; Ou, G.; Du, Y.; He, P.; Zhong, M.; Zhu, H.; Wu, H.; Yang, S.; Liu, Y.; et al. A current collector covering nanostructured villous oxygen-deficient NiO fabricated by rapid laser-scan for Li-O2 batteries. Nano Energy 2018, 51, 83–90. [Google Scholar] [CrossRef]
- Akhtar, N.; Akhtar, W. Prospects, challenges, and latest developments in lithium-air batteries. Int. J. Energy Res. 2014, 39, 303–316. [Google Scholar] [CrossRef]
- Ganesan, P.; Prabu, M.; Sanetuntikul, J.; Shanmugam, S. Cobalt sulfide nanoparticles grown on nitrogen and sulfur codo-ped graphene oxide: An efficient electrocatalyst for oxygen reduction and evolution reactions. ACS Catal. 2015, 5, 3625–3637. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, J.; Yin, R.; Li, B.; Huang, X.; Zhao, L.; Qian, L. Single-atom Pt supported on holey ultrathin g-C3N4 nanosheets as efficient catalyst for Li-O2 batteries. J. Colloid Interface Sci. 2020, 564, 28–36. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, T.; Cheng, F.; Zhao, Q.; Han, X.; Chen, J. Recycling application of Li-MnO2 batteries as rechargeable lithi-um–air batteries. Angew. Chem. Int. Ed. 2015, 54, 4338–4343. [Google Scholar] [CrossRef]
- Ionescu, M.I.; Laforgue, A. Synthesis of nitrogen-doped carbon nanotubes directly on metallic foams as cathode material with high mass load for lithium-air batteries. Thin Solid Films 2020, 709, 138211. [Google Scholar] [CrossRef]
- Luo, H.; Jiang, W.-J.; Zhang, Y.; Niu, S.; Tang, T.; Huang, L.-B.; Chen, Y.-Y.; Wei, Z.; Hu, J.-S. Self-terminated activation for high-yield production of N,P-codoped nanoporous carbon as an efficient metal-free electrocatalyst for Zn-air battery. Carbon 2018, 128, 97–105. [Google Scholar] [CrossRef]
- Li, S.; Hao, X.; Abudula, A.; Guan, G. Nanostructured Co-based bifunctional electrocatalysts for energy conversion and storage: Current status and perspectives. J. Mater. Chem. A 2019, 7, 18674–18707. [Google Scholar] [CrossRef]
- Budnikova, Y.H. Recent advances in metal–organic frameworks for electrocatalytic hydrogen evolution and overall water splitting reactions. Dalton Trans. 2020, 49, 12483–12502. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Jiang, W.J.; Niu, S.; Zhang, X.; Zhang, Y.; Yuan, L.P.; He, C.X.; Hu, J.S. Self-Catalyzed Growth of Co-N-C Nano-brushes for Efficient Rechargeable Zn-Air Batteries. Small 2020, 16, 2001171. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Jaroniec, M.; Qiao, S.Z. Porous C3N4Nanolayers@N-Graphene Films as Catalyst Electrodes for Highly Efficient Hydrogen Evolution. ACS Nano 2015, 9, 931–940. [Google Scholar] [CrossRef]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.-J.; Baek, J.-B. Metal-Free Catalysts for Oxygen Reduction Reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef]
- Hang, Y.; Zhang, C.; Luo, X.; Xie, Y.; Xin, S.; Li, Y.; Zhang, D.W.; Goodenough, J.B. α-MnO2 nanorods supported on porous graphitic carbon nitride as efficient electrocatalysts for lithium-air batteries. J. Power Sources 2018, 392, 15–22. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, T.; Zhang, Y.; Xin, S.; He, X.; Zhang, D.; Shui, J. Electrocatalytic performances of g-C3N4-LaNiO3 composite as bi-functional catalysts for lithium-oxygen batteries. Sci. Rep. 2016, 6, 24314. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Thomas, A.; Fu, X.; Antonietti, M. Metal-containing carbon nitride compounds: A new functional or-ganic-metal hybrid material. Adv. Mater. 2009, 21, 1609–1612. [Google Scholar] [CrossRef]
- Samojlov, A.; Schuster, D.; Kahr, J.; Freunberger, S.A. Surface and catalyst driven singlet oxygen formation in Li-O2 cells. Electrochim. Acta 2020, 362, 137175. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, B.; Zhao, Y.; Tkacheva, A.; Liu, Z.; Yan, K.; Guo, X.; McDonagh, A.M.; Shanmukaraj, D.; Wang, C.; et al. A versatile functionalized ionic liquid to boost the solution-mediated performances of lithium-oxygen batteries. Nat. Commun. 2019, 10, 602. [Google Scholar] [CrossRef]
- Qian, Z.; Li, X.; Sun, B.; Du, L.; Wang, Y.; Zuo, P.; Yin, G.; Zhang, J.; Sun, B.; Wang, G. Unraveling the Promotion Effects of a Soluble Cobaltocene Catalyst with Respect to Li–O2 Battery Discharge. J. Phys. Chem. Lett. 2020, 11, 7028–7034. [Google Scholar] [CrossRef]
- Sano, T.; Tsutsui, S.; Koike, K.; Hirakawa, T.; Teramoto, Y.; Negishi, N.; Takeuchi, K. Activation of graphitic carbon nitride (g-C3N4) by alkaline hydrothermal treatment for photocatalytic NO oxidation in gas phase. J. Mater. Chem. A 2013, 1, 6489–6496. [Google Scholar] [CrossRef]
- Nie, H.; Ou, M.; Zhong, Q.; Zhang, S.; Yu, L. Efficient visible-light photocatalytic oxidation of gaseous NO with graphitic carbon nitride (g–C3N4) activated by the alkaline hydrothermal treatment and mechanism analysis. J. Hazard. Mater. 2015, 300, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Zhao, M.; Ma, Y.; Ye, Z.; Huang, J. Bioinspired Formation of 3D Hierarchical CoFe2O4 Porous Microspheres for Magnetic-Controlled Drug Release. ACS Appl. Mater. Interfaces 2015, 7, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, W.; Chen, Y.; Fan, H.; Su, D.; Wang, G. MOF-derived porous N–Co3O4@N–C nanododecahedra wrapped with reduced graphene oxide as a high capacity cathode for lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 2797–2807. [Google Scholar] [CrossRef]
- Kong, H.J.; Won, D.H.; Kim, J.; Woo, S.I. Sulfur-Doped g-C3N4/BiVO4 Composite Photocatalyst for Water Oxidation under Visible Light. Chem. Mater. 2016, 28, 1318–1324. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.-L.; Chen, Z.; Li, H.; Xiong, B.-Q.; Zhang, P.-L.; Tang, K.-W. Visible-light photoredox-catalyzed dual C–C bond cleavage: Synthesis of 2-cyanoalkylsulfonylated 3,4-dihydronaphthalenes through the insertion of sulfur dioxide. Chem. Commun. 2020, 56, 3011–3014. [Google Scholar] [CrossRef]
- Chen, D.; Dong, C.-L.; Zou, Y.; Su, D.; Huang, Y.-C.; Tao, L.; Dou, S.; Shen, S.; Wang, S. In situ evolution of highly dispersed amorphous CoOx clusters for oxygen evolution reaction. Nanoscale 2017, 9, 11969–11975. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Li, Y.; Sun, L.; Mi, H.; Ren, X.; Zhang, P. Heterostructured CoO-Co3O4 nanoparticles anchored on nitrogen-doped hollow carbon spheres as cathode catalysts for Li-O2 batteries. Nanoscale 2019, 11, 14769–14776. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Han, Q.; Wang, B.; Gao, J.; Cheng, Z.; Zhao, Y.; Zhang, Z.; Qu, L. Atomically Thin Mesoporous Nanomesh of Graphitic C3N4 for High-Efficiency Photocatalytic Hydrogen Evolution. ACS Nano 2016, 10, 2745–2751. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, C.; Qu, X.; Ren, Y.; Yin, J.; Wang, W.; Yang, M.S.; Huang, W.; Dong, X. Sandwich-Structured Fe-Ni2P/MoSx/NF Bifunctional Electrocatalyst for Overall Water Splitting. Adv. Mater. Interfaces 2020, 7, 1901926. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, D.H.; Kang, T.-G.; Ko, Y.; Kang, K.; Lee, Y.J. Bifunctional MnO2-Coated Co3O4 Hetero-structured Catalysts for Reversible Li-O2 Batteries. Chem. Mater. 2017, 29, 10542–10550. [Google Scholar] [CrossRef]
- Wang, J.; Gao, R.; Zhou, D.; Chen, Z.; Wu, Z.; Schumacher, G.; Hu, Z.; Liu, X. Boosting the Electrocatalytic Activity of Co3O4 Nanosheets for a Li-O2 Battery through Modulating Inner Oxygen Vacancy and Exterior Co3+/Co2+ Ratio. ACS Catal. 2017, 7, 6533–6541. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhao, Y.; Ding, L.; Wang, D.; Guo, Q.; Li, Z.; Luo, H.; Zhang, D.; Yu, Y. Enhancing the Capacity and Stability by CoFe2O4 Modified g-C3N4 Composite for Lithium-Oxygen Batteries. Nanomaterials 2021, 11, 1088. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11051088
Li X, Zhao Y, Ding L, Wang D, Guo Q, Li Z, Luo H, Zhang D, Yu Y. Enhancing the Capacity and Stability by CoFe2O4 Modified g-C3N4 Composite for Lithium-Oxygen Batteries. Nanomaterials. 2021; 11(5):1088. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11051088
Chicago/Turabian StyleLi, Xiaoya, Yajun Zhao, Lei Ding, Deqiang Wang, Qi Guo, Zhiwei Li, Hao Luo, Dawei Zhang, and Yan Yu. 2021. "Enhancing the Capacity and Stability by CoFe2O4 Modified g-C3N4 Composite for Lithium-Oxygen Batteries" Nanomaterials 11, no. 5: 1088. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11051088