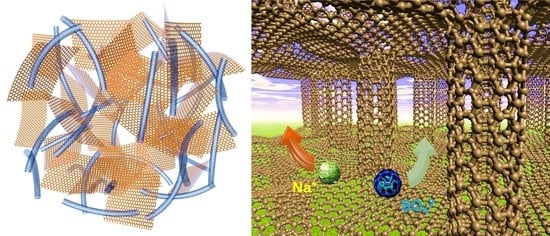
Free-Standing rGO-CNT Nanocomposites with Excellent Rate Capability and Cycling Stability for Na2SO4 Aqueous Electrolyte Supercapacitors
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials Synthesis
2.2. Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clerici, F.; Fontana, M.; Bianco, S.; Serrapede, M.; Perrucci, F.; Ferrero, S.; Tresso, E.; Lamberti, A. In situ MoS2 Decoration of Laser-Induced Graphene as Flexible Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2016, 8, 10459. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, T.Z.; Du, Y.; Dou, S.X.; Zhang, H.; Jiang, L.; Cheng, Q.F. Strong bioinspired HPA-rGO nanocomposite films via interfacial interactions for flexible supercapacitors. Nano Energy 2019, 58, 517. [Google Scholar] [CrossRef]
- Zhou, R.; Han, C.J.; Wang, X.M. Hierarchical MoS2-coated three-dimensional graphene network for enhanced supercapacitor performances. J. Power Sources 2017, 352, 99. [Google Scholar] [CrossRef]
- Saraf, M.; Natarajan, K.; Mobin, S.M. Emerging Robust Heterostructure of MoS2–rGO for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 16588. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Niu, Z.Q.; Chen, J. Flexible supercapacitors based on carbon nanotubes. Chin. Chem. Lett. 2018, 29, 571. [Google Scholar] [CrossRef]
- Guo, D.X.; Song, X.M.; Tan, L.C.; Ma, H.Y.; Sun, W.F.; Pang, H.J.; Zhang, L.L.; Wang, X.M. A facile dissolved and reassembled strategy towards sandwich-like rGO@NiCoAl-LDHs with excellent supercapacitor performance. Chem. Eng. J. 2019, 356, 955. [Google Scholar] [CrossRef]
- Kumar, K.S.; Choudhary, N.; Jung, Y.; Thomas, J. Recent Advances in Two-Dimensional Nanomaterials for Supercapacitor Electrode Applications. ACS Energy Lett. 2018, 3, 482. [Google Scholar] [CrossRef]
- Qu, C.; Zhao, B.T.; Jiao, Y.; Chen, D.C.; Dai, S.G.; Deglee, B.M.; Chen, Y.; Walton, K.S.; Zou, R.Q.; Liu, M.L. Functionalized Bimetallic Hydroxides Derived from Metal–Organic Frameworks for High-Performance Hybrid Supercapacitor with Exceptional Cycling Stability. ACS Energy Lett. 2017, 2, 1263. [Google Scholar] [CrossRef]
- Zeng, X.J.; Yang, B.; Li, X.P.; Yu, R.H. Three-dimensional hollow CoS2 nanoframes fabricated by anion replacement and their enhanced pseudocapacitive performances. Electrochim. Acta 2017, 240, 341. [Google Scholar] [CrossRef]
- Patil, S.J.; Kim, J.H.; Lee, D.W. Graphene-nanosheet wrapped cobalt sulphide as a binder free hybrid electrode for asymmetric solid-state supercapacitor. J. Power Sources 2017, 342, 652. [Google Scholar] [CrossRef]
- Li, Z.J.; Zhang, W.Y.; Sun, C.Y.; Feng, Z.F.; Yang, B.C. Controlled synthesis of Ni(OH)2/graphene composites and their transformation to NiO/graphene for energy storage. Electrochim. Acta 2016, 212, 390. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Ahmed, B.; Anjum, D.; Alshareef, H.N. Direct Chemical Synthesis of MnO2 Nanowhiskers on Transition-Metal Carbide Surfaces for Supercapacitor Applications. ACS Appl. Mater. Interfaces 2016, 8, 18806. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Zhang, P.; Chen, J.; Tian, W.B.; Zhang, Y.M.; Sun, Z.M. In situ synthesis of CNTs@Ti3C2 hybrid structures by microwave irradiation for high-performance anodes in lithium ion batteries. J. Mater. Chem. A 2018, 6, 3543. [Google Scholar] [CrossRef]
- Liu, P.B.; Yan, J.; Guang, Z.X.; Huang, Y.; Li, X.F.; Huang, W.H. Recent advancements of polyaniline-based nanocomposites for supercapacitors. J. Power Sources 2019, 424, 108. [Google Scholar] [CrossRef]
- Strauss, V.; Marsh, K.; Kowal, M.D.; El-Kady, M.; Kaner, R.B. A Simple Route to Porous Graphene from Carbon Nanodots for Supercapacitor Applications. Adv. Mater. 2018, 30, 1704449. [Google Scholar] [CrossRef]
- Yao, L.; Wu, Q.; Zhang, P.X.; Zhang, J.M.; Wang, D.R.; Li, Y.L.; Ren, X.Z.; Mi, H.W.; Deng, L.B.; Zheng, Z.J. Scalable 2D Hierarchical Porous Carbon Nanosheets for Flexible Supercapacitors with Ultrahigh Energy Density. Adv. Mater. 2018, 30, 1706054. [Google Scholar] [CrossRef]
- Yang, Z.F.; Tian, J.R.; Yin, Z.F.; Cui, C.J.; Qian, W.Z.; Wei, F. Carbon nanotube- and graphene-based nanomaterials and applications in high-voltage supercapacitor: A review. Carbon 2019, 141, 467. [Google Scholar] [CrossRef]
- Liu, C.F.; Liu, Y.C.; Yi, T.Y.; Hu, C.C. Carbon materials for high-voltage supercapacitors. Carbon 2018, 145, 529. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.Y.; You, W.; Yu, J.G. Core–Shell Nitrogen-Doped Carbon Hollow Spheres/Co3O4 Nanosheets as Advanced Electrode for High-Performance Supercapacitor. Small 2018, 14, 1702407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Kang, H.W.; Liu, H.L.; Yang, B.C.; Liu, Y.X.; Yuan, M.K.; Li, Z.K. 1-Aminopyrene/rGO nanocomposites for electrochemical energy storage with improved performance. Chem. Eng. J. 2019, 361, 189. [Google Scholar] [CrossRef]
- Hao, P.; Zhao, Z.H.; Leng, Y.H.; Tian, J.; Sang, Y.H.; Boughton, R.I.; Wong, C.P.; Liu, H.; Yang, B. Graphene-based nitrogen self-doped hierarchical porous carbon aerogels derived from chitosan for high performance supercapacitors. Nano Energy 2015, 15, 9. [Google Scholar] [CrossRef]
- Li, B.; Dai, F.; Xiao, Q.F.; Yang, L.; Shen, J.M.; Zhang, C.M.; Cai, M. Nitrogen-doped activated carbon for a high energy hybrid supercapacitor. Energy Environ. Sci. 2016, 9, 102. [Google Scholar] [CrossRef]
- Shi, Q.R.; Cha, Y.; Song, Y.; Lee, J.I.; Zhu, C.Z.; Li, X.Y.; Song, M.K.; Du, D.; Lin, Y.H. 3D graphene-based hybrid materials: Synthesis and applications in energy storage and conversion. Nanoscale 2016, 8, 15414. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.I.; Yoon, J.C.; Jang, J.H. Chemical Vapor Deposition of Mesoporous Graphene Nanoballs for Supercapacitor. ACS Nano 2013, 7, 6047. [Google Scholar] [CrossRef]
- Li, H.; Tao, Y.; Zheng, X.Y.; Luo, J.Y.; Kang, F.Y.; Cheng, H.M.; Yang, Q.H. Ultra-thick graphene bulk supercapacitor electrodes for compact energy storage. Energy Environ. Sci. 2016, 9, 3135. [Google Scholar] [CrossRef]
- Wang, B.H.; Qin, Y.; Tan, W.S.; Tao, Y.X.; Kong, Y. Smartly designed 3D N-doped mesoporous graphene for high-performance supercapacitor electrodes. Electrochim. Acta 2017, 241, 1. [Google Scholar] [CrossRef]
- Song, B.; Zhao, J.X.; Wang, M.J.; Mullavey, J.; Zhu, Y.T.; Geng, Z.S.; Chen, D.C.; Ding, Y.; Moon, K.S.; Liu, M.L.; et al. Systematic study on structural and electronic properties of diamine/triamine functionalized graphene networks for supercapacitor application. Nano Energy 2017, 31, 183. [Google Scholar] [CrossRef]
- Down, M.P.; Rowley-Neale, S.J.; Smith, G.C.; Banks, C.E. Fabrication of Graphene Oxide Supercapacitor Devices. ACS Appl. Energy Mater. 2018, 1, 707. [Google Scholar] [CrossRef] [Green Version]
- Couly, C.M.; Alhabeb, K.L.; Van Aken, N.; Kurra, L.; Gomes, A.M.; Alshareef, H.N.; Gogotsi, Y. Asymmetric Flexible MXene-Reduced Graphene Oxide Micro-Supercapacitor. Adv. Electron. Mater. 2018, 4, 1700339. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.W.; Yu, H.T.; Xing, R.G.; Ge, X.; Sun, H.; Li, R.H.; Zhang, Q.W. Multi-growth site graphene/polyaniline composites with highly enhanced specific capacitance and rate capability for supercapacitor application. Electrochim. Acta 2018, 260, 504. [Google Scholar] [CrossRef]
- Yang, S.H.; Liu, Y.Y.; Hao, Y.F.; Yang, X.P.; Goddard, W.A., III; Zhang, X.L.; Cao, B.Q. Oxygen-Vacancy Abundant Ultrafine Co3O4/Graphene Composites for High-Rate Supercapacitor Electrodes. Adv. Sci. 2018, 5, 1700659. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Li, J.Y.; Feng, Y.P. Carbon Nanotubes for Supercapacitor. Nanoscale Res. Lett. 2010, 5, 654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frackowiak, E.; Metenier, K.; Bertagna, V.; Beguin, F. Supercapacitor electrodes from multiwalled carbon nanotubes. Appl. Phys. Lett. 2000, 77, 2421. [Google Scholar] [CrossRef]
- Ding, B.; Guo, D.; Wang, Y.H.; Wu, X.L.; Fan, Z.J. Functionalized graphene nanosheets decorated on carbon nanotubes networks for high performance supercapacitors. J. Power Sources 2018, 398, 113. [Google Scholar] [CrossRef]
- Bi, W.C.; Gao, G.H.; Wu, Y.J.; Yang, H.Y.; Wang, J.C.; Zhang, Y.R.; Liang, X.; Liu, Y.D.; Wu, G.M. Novel three-dimensional island-chain structured V2O5/graphene/MWCNT hybrid aerogels for supercapacitors with ultralong cycle life. RSC Adv. 2017, 7, 7179. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.S.; Zhao, N.; Wang, H.; Xu, J.; Pan, F. 3D conductive network-based free-standing PANI–RGO–MWNTs hybrid film for high-performance flexible supercapacitor. J. Mater. Chem. A 2014, 2, 12340. [Google Scholar] [CrossRef]
- Zhu, J.X.; Yang, D.; Yin, Z.Y.; Yan, Q.Y.; Zhang, H. Graphene and Graphene-Based Materials for Energy Storage Applications. Small 2014, 10, 3480. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kumar, K.; Fisher, F.T.; Yang, E.H. Out-of-plane growth of CNTs on graphene for supercapacitor applications. Nanotechnology 2011, 23, 015301. [Google Scholar] [CrossRef]
- Kim, S.I.; Thiyagarajan, P.; Jang, J.H. Great improvement in pseudocapacitor properties of nickel hydroxide via simple gold deposition. Nanoscale 2014, 6, 11646. [Google Scholar] [CrossRef]
- Tran, D.; Kabiri, S.; Losic, D. A green approach for the reduction of graphene oxide nanosheets using non-aromatic amino acids. Carbon 2014, 76, 193. [Google Scholar] [CrossRef]
- Ammar, M.R.; Galy, N.; Rouzaud, J.N.; Toulhoat, N.; Vaudey, C.E.; Simon, P.; Moncoffre, N. Characterizing various types of defects in nuclear graphite using Raman scattering: Heat treatment, ion irradiation and polishing. Carbon 2015, 95, 364. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Chen, L.; Liu, H.L.; Kang, H.W.; Zhang, S.R.; Yang, B.C.; Wang, Y.; Yuan, M.K.; Li, Z.K. High capacitive and super-long life supercapacitor fabricated by 7-aminoindole/reduced graphene oxide composite. Electrochim. Acta 2019, 332, 135528. [Google Scholar] [CrossRef]
- Jeong, H.M.; Lee, J.W.; Shin, W.H.; Choi, Y.J.; Shin, H.J.; Kang, J.K.; Choi, J.W. Nitrogen-Doped Graphene for High-Performance Ultracapacitors and the Importance of Nitrogen-Doped Sites at Basal Planes. Nano Lett. 2011, 11, 2472. [Google Scholar] [CrossRef]
- Hu, T.; Liu, Y.Y.; Zhang, Y.F.; Chen, M.; Zheng, J.Q.; Tang, J.; Meng, C.G. 3D hierarchical porous V3O7·H2O nanobelts/CNT/reduced graphene oxide integrated composite with synergistic effect for supercapacitors with high capacitance and long cycling life. J. Colloid Interface Sci. 2018, 531, 382. [Google Scholar] [CrossRef] [PubMed]
- Lytle, J.C.; Wallace, J.M.; Sassin, M.B.; Barrow, A.J.; Long, J.W.; Dysart, J.L.; Renninger, C.H.; Saunders, M.P.; Brandell, N.L.; Rolison, D.R. The right kind of interior for multifunctional electrode architectures: Carbon nanofoam papers with aperiodic submicrometre pore networks interconnected in 3D. Energy Environ. Sci. 2011, 4, 1913. [Google Scholar] [CrossRef]
- Hua, W.B.; Guo, X.D.; Zheng, Z.; Wang, Y.J.; Zhong, B.H.; Fang, B.Z.; Wang, J.Z.; Chou, S.L.; Liu, H. Uncovering a facile large-scale synthesis of LiNi1/3Co1/3Mn1/3O2 nanoflowers for high power lithium-ion batteries. J. Power Sources 2015, 275, 200. [Google Scholar] [CrossRef]
- Fang, B.Z.; Kim, M.S.; Kim, J.H.; Lim, S.; Yu, J.S. Ordered multimodal porous carbon with hierarchical nanostructure for high Listorage capacity and good cycling performance. J. Mater. Chem. 2010, 20, 10253. [Google Scholar] [CrossRef]
- Mohammadi, A.; Arsalani, N.; Tabrizi, A.G.; Moosavifard, S.E.; Naqshbandi, Z.; Ghadimib, L.S. Engineering rGO-CNT wrapped Co3S4 nanocomposites for high-performance asymmetric supercapacitors. Chem. Eng. J. 2018, 334, 66. [Google Scholar]
- Li, X.; Tang, Y.; Song, J.H.; Yang, W.; Wang, M.S.; Zhu, C.Z.; Zhao, W.G.; Zheng, J.M.; Lin, Y.H. Self-supporting activated carbon/carbon nanotube/reduced graphene oxide flexible electrode for high performance supercapacitor. Carbon 2018, 129, 236. [Google Scholar] [CrossRef]
- Shi, K.Y.; Zhitomirsky, I. Polypyrrole nanofiber–carbon nanotube electrodes for supercapacitors with high mass loading obtained using an organic dye as a co-dispersant. J. Mater. Chem. A 2013, 1, 11614. [Google Scholar] [CrossRef]
- Yang, L.; Zheng, W.; Zhang, P.; Chen, J.; Tian, W.B.; Zhang, Y.M.; Sun, Z.M. MXene/CNTs films prepared by electrophoretic deposition for supercapacitor electrodes. J. Electroanal. Chem. 2018, 830, 1. [Google Scholar] [CrossRef]
- Miao, F.J.; Shao, C.L.; Li, X.H.; Lu, N.; Wang, K.X.; Zhang, X.; Liu, Y.C. Polyaniline-coated electrospun carbon nanofibers with high mass loading and enhanced capacitive performance as freestanding electrodes for flexible solid-state supercapacitors. Energy 2016, 95, 233. [Google Scholar] [CrossRef]
- Yu, C.J.; Masarapu, C.; Rong, J.P.; Wei, B.Q.; Jiang, H.Q. Stretchable Supercapacitors Based on Buckled Single-Walled Carbon-Nanotube Macrofilms. Adv. Mater. 2009, 21, 4793. [Google Scholar] [CrossRef]
- Pham, D.T.; Lee, T.H.; Luong, D.H.; Yao, F.; Ghosh, A.; Le, V.T.; Kim, T.H.; Li, B.; Chang, J.; Lee, Y.H. Dual-Gated MoS2/WSe2 van der Waals Tunnel Diodes and Transistors. ACS Nano 2015, 9, 2017. [Google Scholar]
- Saravanakumar, B.; Purushothaman, K.K.; Muralidharan, G. V2O5/functionalized MWCNT hybrid nanocomposite: The fabrication and its enhanced supercapacitive performance. RSC Adv. 2014, 4, 37437. [Google Scholar] [CrossRef]
- Zhu, J.B.; Xu, Y.L.; Wang, J.; Wang, J.P.; Bai, Y.; Du, X.F. Morphology controllable nano-sheet polypyrrole–graphene composites for high-rate supercapacitor. Phys. Chem. Chem. Phys. 2015, 17, 19885. [Google Scholar] [CrossRef] [PubMed]
- Teo, E.; Hong, N.L.; Jose, R.; Chong, K.F. Aminopyrene functionalized reduced graphene oxide as a supercapacitor electrode. RSC Adv. 2015, 5, 38111. [Google Scholar] [CrossRef] [Green Version]
- Malik, R.; Zhang, L.; Mcconnell, C.; Schott, M.; Hsieh, Y.Y.; Noga, R.; Alvarez, N.T.; Shanov, V. Three-dimensional, free-standing polyaniline/carbon nanotube composite-based electrode for high-performance supercapacitors. Carbon 2017, 116, 579. [Google Scholar] [CrossRef]
- Huang, Y.P.; Zhou, J.J.; Gao, N.; Yin, Z.X.; Zhou, H.H.; Yang, X.Y.; Kuang, Y.F. Synthesis of 3D reduced graphene oxide/unzipped carbon nanotubes/polyaniline composite for high-performance supercapacitors. Electrochim. Acta 2018, 269, 649. [Google Scholar] [CrossRef]
- Patil, S.S.; Dubal, D.P.; Deonikar, V.G.; Tamboli, M.S.; Ambekar, J.D.; Gomez-Romero, P.; Kolekar, S.S.; Kale, B.B.; Patil, D.R. Fern-like rGO/BiVO4 Hybrid Nanostructures for High-Energy Symmetric Supercapacitor. ACS Appl. Mater. Interfaces 2016, 8, 31602. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.B.; Xu, C.L.; Yu, D.M.; Chen, C.G. Pseudocapacitance multiporous vanadyl phosphate/graphene thin film electrode for high performance electrochemical capacitors. J. Colloid Interface Sci. 2021, 590, 341. [Google Scholar] [CrossRef]









| Sample | D-Band (cm−1) | G-Band (cm−1) | ID/IG |
|---|---|---|---|
| CNT | 1329 | 1592.4 | 1.25 |
| GO | 1336.2 | 1592.8 | 1.12 |
| rGO | 1331.1 | 1593.4 | 1.23 |
| rGO-CNT10 | 1333.3 | 1589.3 | 1.27 |
| rGO-CNT20 | 1332.2 | 1594.5 | 1.26 |
| rGO-CNT30 | 1334.4 | 1592.4 | 1.27 |
| rGO-CNT50 | 1330.1 | 1594.5 | 1.29 |
| Samples | Specific Capacitance (F g−1) | Current Density/Scan Rate | Energy Density (W h kg−1) /Power Density (W kg−1) | Capacitance Retention (Cycle Numbers) | Ref. |
|---|---|---|---|---|---|
| G/GNTs-200 | 202 | 0.5 A g−1 | 11.7/50 | 102% (20,000) | [34] |
| Ti3C2/CNTs | 134 | 1 A g−1 | 2.77/311 | >100% (10,000) | [51] |
| PPy-RGO | 255.7 | 0.2 A g−1 | 7.02/89 | 93% 1000 | [56] |
| Ap-rGO | 160 | 5 mV s−1 | 5.6/50 | 85% (5000) | [57] |
| Polyaniline/ Carbon Nanotube | 359 | 1.56 mA cm-2 | 11.1/980 | 92% (10,000) | [58] |
| RGO/UCNTs/PANI | 359.3 | 1 A g−1 | 7.4/189 | 80.5% (2000) | [59] |
| rGO/BiVO4 | 196 | 5 mV s−1 | 33.7/1140 and 15.33/8000 | 80.3% (2000) | [60] |
| MP-VOPO4@rGO | 672 | 1 A g−1 | 26.3/250 and 10.1/5000 | 83.5% (5000) | [61] |
| rGO-CNT30 | 241.62 | 0.5 A g−1 | 12.29/425 and 9.56/8500 | 91.42% (18,000) | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.; Qin, Z.; Li, Z. Free-Standing rGO-CNT Nanocomposites with Excellent Rate Capability and Cycling Stability for Na2SO4 Aqueous Electrolyte Supercapacitors. Nanomaterials 2021, 11, 1420. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11061420
Du X, Qin Z, Li Z. Free-Standing rGO-CNT Nanocomposites with Excellent Rate Capability and Cycling Stability for Na2SO4 Aqueous Electrolyte Supercapacitors. Nanomaterials. 2021; 11(6):1420. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11061420
Chicago/Turabian StyleDu, Xiaohan, Zhen Qin, and Zijiong Li. 2021. "Free-Standing rGO-CNT Nanocomposites with Excellent Rate Capability and Cycling Stability for Na2SO4 Aqueous Electrolyte Supercapacitors" Nanomaterials 11, no. 6: 1420. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11061420