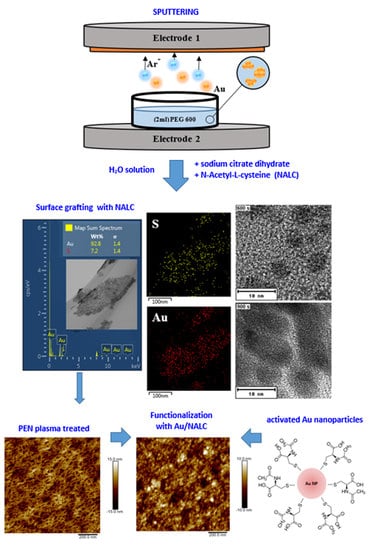
PEGylated Gold Nanoparticles Grafted with N-Acetyl-L-Cysteine for Polymer Modification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Techniques
3. Results
3.1. Stability Studies of AuNP Solutions in Sodium Citrate Dihydrate
3.2. Study of AuNP Stability in N-Acetyl-L-Cysteine
3.3. Modification of PEN Using AuNP/PEG/NALC/H2O
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jamkhande, P.G.; Ghule, N.W.; HaqueBamer, A.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Matsuyama, K.; Tsubaki, T.; Kato, T.; Okuyama, T.; Muto, H. Preparation of catalytically active Au nanoparticles by sputter deposition and their encapsulation in metal-organic framework of Cu3(BTC)2. Mater. Lett. 2020, 261, 127124. [Google Scholar] [CrossRef]
- Slepička, P.; Slepičková Kasálková, N.; Siegel, J.; Kolská, Z.; Švorčík, V. Methods of Gold and Silver Nanoparticles Preparation. Materials 2020, 13, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, M.; Yamamoto, M.; Sakata, M. Temperature dependence on the size control of palladium nanoparticles by chemical reduction in nonionic surfactant/ionic liquid hybrid systems. J. Mol. Liquids 2020, 311, 113255. [Google Scholar] [CrossRef]
- De Souza, C.D.; Ribeiro Nogueira, B.; Rostelato, M.E.C.M. Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. J. Alloys Compound. 2019, 798, 714–740. [Google Scholar] [CrossRef]
- Machida, H.; Sugahara, T.; Hirasawa, I. Preparation of dispersed metal nanoparticles in the aqueous solution of metal carboxylate and the tetra-n-butylammonium carboxylate. J. Crystal Growth 2019, 514, 14–20. [Google Scholar] [CrossRef]
- Zhou, J.; Ralston, J.; Sedev, R.; Beattie, D.A. Functionalized gold nanoparticles: Synthesis, structure and colloid stability. J. Colloid Interface Sci. 2008, 331, 251–262. [Google Scholar] [CrossRef]
- Wagener, M.; Günther, B. Sputtering on liquids—A versatile process for the production of magnetic suspensions? J. Magn. Magn. Mater. 1999, 201, 41–44. [Google Scholar] [CrossRef]
- Torimoto, T.; Okazaki, K. Sputter deposition onto ionic liquids: Simple and clean synthesis of highly dispersed ultrafine metal nanoparticles. Appl. Phys. Lett. 2006, 89, 24311. [Google Scholar] [CrossRef]
- Ye, G.X.; Zhang, Q.R.; Feng, C.M.; Ge, H.L.; Jiao, Z.K. Structural and electrical properties of a metallic rough-thin-film system deposited on liquid substrates. Phys. Rev. B Condens. Matter 1996, 54, 14754–14757. [Google Scholar]
- Slepička, P.; Elashnikov, R.; Ulbrich, P.; Staszek, M.; Kolská, Z.; Švorčík, V. Stabilization of sputtered gold and silver nanoparticles in PEG colloid solutions. J. Nanopart. Res. 2015, 17, 11–26. [Google Scholar] [CrossRef]
- Slepička, P.; Přibyl, M.; Fajstavr, D.; Ulbrich, P.; Siegel, J.; Řezníčková, A.; Švorčík, V. Grafting of platinum nanostructures on biopolymer at elevated temperature. Colloids Surf. A 2018, 546, 316–325. [Google Scholar] [CrossRef]
- Reznickova, A.; Slepicka, P.; Slavikova, N.; Staszek, M.; Svorcik, V. Preparation, aging and temperature stability of PEGylated gold nanoparticles. Colloids Surf. A 2017, 523, 91–97. [Google Scholar] [CrossRef]
- Parveen, R.; Ullah, S.; Sgarbi, R.; Tremiliosi-Filho, G. One-pot ligand-free synthesis of gold nanoparticles: The role of glycerol as reducing-cum-stabilizing agent. Colloids Surf. A 2019, 565, 162–171. [Google Scholar] [CrossRef]
- Leopold, N.; Chiş, V.; Mircescu, N.E.; Marişca, O.T.; Buja, O.M.; Leopold, L.F.; Socaciu, C.; Braicu, C.; Irimie, A.; Berindan-Neagoe, I. One step synthesis of SERS active colloidal gold nanoparticles by reduction with polyethylene glycol. Colloids Surf. A 2013, 436, 133–138. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, L.; Fu, D.; Zhao, D.; Wang, Y.; Yuan, Q.; Zhu, Y.; Yang, J.; Yang, F. Gold nanoparticles amplified microcantilever biosensor for detecting protein biomarkers with high sensitivity. Sens. Actuators A 2021, 321, 112563. [Google Scholar] [CrossRef]
- Hua, Z.; Yu, T.; Liu, D.; Xianyu, Y. Recent advances in gold nanoparticles-based biosensors for food safety detection. Biosensors Bioelectron. 2021, 179, 113076. [Google Scholar] [CrossRef] [PubMed]
- Suárez-García, S.; Solórzano, R.; Novio, F.; Alibés, R.; Busqué, F.; Ruiz-Molina, D. Coordination polymers nanoparticles for bioimaging. Coord. Chem. Rev. 2021, 432, 213716. [Google Scholar] [CrossRef]
- Wang, Y.; Langley, R.J.; Tamshen, K.; Harms, J.; Middleditch, M.J.; Maynard, H.D.; Jamieson, S.M.F.; Perry, J.K. Enhanced Bioactivity of a Human GHR Antagonist Generated by Solid-Phase Site-Specific PEGylation. Biomacromolecules 2021, 22, 299–308. [Google Scholar] [CrossRef]
- Matsuhira, T.; Sakai, H. Entropy-Driven Supramolecular Ring-Opening Polymerization of a Cyclic Hemoglobin Monomer for Constructing a Hemoglobin–PEG Alternating Polymer with Structural Regularity. Biomacromolecules 2021, 22, 1944–1954. [Google Scholar] [CrossRef]
- Qian, W.; Murakami, M.; Ichikawa, Y.; Che, Y. Highly efficient and controllable PEGylation of gold nanoparticles prepared by femtosecond laser ablation in water. J. Phys. Chem. C 2011, 115, 23293–23298. [Google Scholar]
- Takae, S.; Akiyama, Y.; Otsuka, H.; Nakamura, T.; Nagasaki, Y.; Kataoka, K. Ligand density effect on biorecognition by PEGylated gold nanoparticles: Regulated interaction of RCA120 lectin with lactose installed to the distal end of tethered PEG strands on gold surface. Biomacromolecules 2005, 6, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Shimmin, R.G.; Schoch, A.B.; Braun, P.V. Polymer size and concentration of effects on the size of gold nanoparticles cappped by polymeric thiols. Langmuir 2007, 20, 5613–5620. [Google Scholar] [CrossRef]
- Shenoy, D.; Fu, W.; Li, J.; Crasto, C.; Jones, G.; DiMarzio, C.; Amiji, M. Surface functionalization of gold nanoparticles using hetero-bifunctional poly (ethylene glycol) spacer for intracellular tracking and delivery. Int. J. Nanomed. 2006, 1, 51–57. [Google Scholar] [CrossRef]
- Valkenier, H.; Malytskyi, V.; Blond, P.; Retout, M.; Mattiuzzi, A.; Goole, J.; Raussens, V.; Jabin, I.; Bruylants, G. Orcid Controlled Functionalization of Gold Nanoparticles with Mixtures of Calix[4]arenes Revealed by Infrared Spectroscopy. Langmuir 2017, 33, 8253–8259. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Shenoy, D.; Li, J.; Crasto, C.; Jones, G.; Dimarzio, C.; Sridhar, S.; Amiji, M. Biomedical applications of gold nanoparticles functionalized using hetero-bifunctional poly(ethylene glycol) spacer. MRS Online Proc. Libr. 2004, 1, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Wangoo, N.; Bhasin, K.; Mehta, S.; Suri, C. Synthesis and capping of water-dispersed gold nanoparticles by an amino acid: Bioconjugation and binding studies. J. Colloids. Interface Sci. 2008, 323, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Russier-Antoine, I.; Bertorelle, F.; Kulesza, A.; Soleilhac, A.; Bensalah-Ledoux, A.; Guy, S.; Dugourd, P.; Brevet, P.; Antoine, R. Chiral supramolecular gold-cysteine nanoparticles: Chiroptical and nonlinear optical properties. Prog. Natur. Sci. Mater. Int. 2016, 26, 455–460. [Google Scholar] [CrossRef] [Green Version]
- Sun, L. Functional Gold Nanoparticle−Peptide Complexes as Cell-Targeting Agents. Langmuir 2008, 24, 10293–10297. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, W.; Ge, J.; Zhao, X.S. Kinetics and thermodynamics of DNA hybridization on gold nanoparticles. Nucl. Acid Res. 2009, 37, 3756–3765. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.L.; Tsai, C.Y.; Sun, C.C.; Uppala, R.; Chen, C.C.; Lin, C.H.; Chen, P.H. Electrical detection of DNA using gold and magnetic nanoparticles and bio bar-code DNA between nanogap electrodes. Microelectron. Eng. 2006, 83, 1630–1633. [Google Scholar] [CrossRef]
- Lipka, J.; Semmler-Behnke, M.; Sperling, R.A.; Wenk, A.; Takenaka, S.; Schleh, C.; Kissel, T.; Parak, W.J.; Kreyling, W.G. Biodistribution of PEG-modified gold nanoparticles following intratracheal instillation and intravenous injection. Biomaterials 2010, 31, 6574–6581. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.S.; Cho, M.; Jeong, J.; Choi, M.; Han, B.S.; Shin, H.S.; Hong, J.; Chung, B.H.; Jeong, J.; Cho, M.H. Size-dependent tissue kinetics of PEG-coated gold nanoparticles. Toxicol. Appl. Pharmacol. 2010, 245, 116–123. [Google Scholar] [CrossRef]
- Kolska, Z.; Valha, P.; Slepička, P.; Švorčík, V. Refractometric study of systems water-poly (ethylene glycol) for preparation and characterization of Au nanoparticles dispersion. Arabian J. Chem. 2019, 12, 5019–5027. [Google Scholar] [CrossRef] [Green Version]
- Ishii, T.; Otsuka, H.; Kataoka, K.; Nagasaki, Y. Preparation of functionally PEGylated gold nanoparticles with narrow distribution through autoreduction of auric cation by alpha-biotinyl-PEG-block-[poly(2-N, N-dimethylamino)ethyl methacrylate)]. Langmuir 2004, 20, 561–564. [Google Scholar] [CrossRef]
- Lee, S.H.; Bae, K.H.; Kim, S.H.; Lee, K.R.; Park, T.G. Amine functionalized gold nanoparticles as non-cytotoxic and efficient intracellular siRNA delivery carriers. Int. J. Pharm. 2008, 364, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.A.; Kim, J.P.; Oyama, M. Preparation of monodispersed carboxylate-functionalized gold nanoparticles using pamoic acid as a reducingand capping reagent. Gold Bull. 2014, 47, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Selegård, R.; Ailic, D.; Liedberg, B. Peptide functionalized gold nanoparticles for colorimetric detection of matrilysin (MMP-7) activity. Nanoscale 2013, 5, 8973–8976. [Google Scholar] [CrossRef]
- Bastis, N.G.; Sanchez-Tillo, E.; Pujals, S.; Farrera, C.; Kogan, M.J.; Giralt, E.; Celada, A.; Iloberas, J.; Puntes, V. Peptides conjugated to gold nanoparticles induce macrophage activation. Mol. Immunol. 2009, 46, 743–748. [Google Scholar] [CrossRef]
- Javier, D.J.; Nitin, N.; Levy, M.; Ellington, A.; Richards-Kortum, R. Aptamer-targeted gold nanoparticles as molecular specific contrast agents for refelectance imaging. Bioconjugate Chem. 2008, 19, 1309–1312. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Jang, H.H.; Ryou, S.M.; Kim, S.; Bae, J.; Lee, K.; Han, M.S. A functionalized gold nanoparticles-assisted universal carrier for antisense DNA. Chem. Commun. 2010, 46, 4151–4153. [Google Scholar] [CrossRef]
- Neděla, O.; Slepička, P.; Švorčík, V. Surface Modification of Polymer Substrates for Biomedical Applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef]
- Slepicka, P.; Slepickova Kasalkova, N.; Siegel, J.; Kolska, Z.; Bacakova, L.; Svorcik, V. Nano-structured and functionalized surfaces for cytocompatibility improvement and bactericidal action. Biotechnol. Adv. 2015, 33, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Slepicka, P.; Siegel, J.; Lyutakov, O.; Slepickova Kasalkova, N.; Kolska, Z.; Bacakova, L.; Svorcik, V. Polymer nanostructures for bioapplications induced by laser treatment. Biotechnol. Adv. 2018, 36, 839–855. [Google Scholar]
- Slepička, P.; Malá, Z.; Rimpelová, S.; Švorčík, V. Antibacterial properties of modified biodegradable PHB non-woven fabric. Mater. Sci. Eng. C 2016, 65, 364–368. [Google Scholar] [CrossRef]












| Sample | Element Concentration (at%) | |
|---|---|---|
| S | Au | |
| Pristin PEN; PEN modif. 200 s; PEN modif. 400 s | - | - |
| PEN modif. 200 s, AuNP 600 s | 1.26 | 0.12 |
| PEN modif. 200 s, AuNP 900 s | 1.26 | 2.32 |
| PEN modif. 400 s, AuNP 600 s | 1.96 | 2.61 |
| PEN modif. 400 s, AuNP 900 s | 0.83 | 0.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fajstavr, D.; Karasová, A.; Michalcová, A.; Ulbrich, P.; Slepičková Kasálková, N.; Siegel, J.; Švorčík, V.; Slepička, P. PEGylated Gold Nanoparticles Grafted with N-Acetyl-L-Cysteine for Polymer Modification. Nanomaterials 2021, 11, 1434. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11061434
Fajstavr D, Karasová A, Michalcová A, Ulbrich P, Slepičková Kasálková N, Siegel J, Švorčík V, Slepička P. PEGylated Gold Nanoparticles Grafted with N-Acetyl-L-Cysteine for Polymer Modification. Nanomaterials. 2021; 11(6):1434. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11061434
Chicago/Turabian StyleFajstavr, Dominik, Adéla Karasová, Alena Michalcová, Pavel Ulbrich, Nikola Slepičková Kasálková, Jakub Siegel, Václav Švorčík, and Petr Slepička. 2021. "PEGylated Gold Nanoparticles Grafted with N-Acetyl-L-Cysteine for Polymer Modification" Nanomaterials 11, no. 6: 1434. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11061434