Tungsten-Modulated Molybdenum Selenide/Graphene Heterostructure as an Advanced Electrode for All-Solid-State Supercapacitors
Abstract
:1. Introduction
2. Experimental Methods
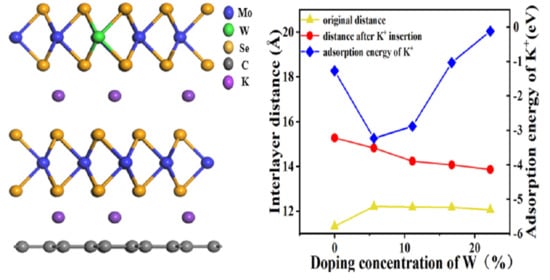
2.1. Density Functional Theory
2.2. Growth of Graphene
2.3. Preparation of a W-Doped MoSe2/Graphene Heterostructure and W-Doped MoSe2 Powder
2.4. Characterization of Materials
2.5. Preparation of the Electrolyte
2.6. Electrochemical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turner, J.A. A realizable renewable energy future. Science 1999, 285, 687–689. [Google Scholar] [CrossRef] [Green Version]
- Lund, H. Renewable energy strategies for sustainable development. Energy 2007, 32, 912–919. [Google Scholar] [CrossRef] [Green Version]
- Dincer, I. Renewable energy and sustainable development: A crucial review. Renew. Sustain. Energy Rev. 2000, 4, 157–175. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor devices based on graphene materials. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Frackowiak, E. Carbon materials for supercapacitor application. Phys. Chem. Chem. Phys. 2007, 9, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Cherusseri, J.; Choudhary, N.; Sambath Kumar, K.S.; Jung, Y.; Thomas, J. Recent trends in transition metal dichalcogenide based supercapacitor electrodes. Nanoscale Horiz. 2019, 4, 840–858. [Google Scholar] [CrossRef]
- Seman, R.N.A.R.; Azam, M.A.; Ani, M.H. Graphene/transition metal dichalcogenides hybrid supercapacitor electrode: Status, challenges, and perspectives. Nanotechnology 2018, 29, 502001. [Google Scholar] [CrossRef]
- Lin, L.; Lei, W.; Zhang, S.; Liu, Y.; Wallace, G.G.; Chen, J. Two-dimensional transition metal dichalcogenides in supercapacitors and secondary batteries. Energy Storage Mater. 2019, 19, 408–423. [Google Scholar] [CrossRef]
- Balasingam, S.K.; Lee, J.S.; Jun, Y. Few-layered MoSe2 nanosheets as an advanced electrode material for supercapacitors. Dalton Trans. 2015, 44, 15491–15498. [Google Scholar] [CrossRef]
- Zhao, X.; Cai, W.; Yang, Y.; Song, X.; Neale, Z.; Wang, H.; Sui, J.; Cao, G. MoSe2 nanosheets perpendicularly grown on graphene with Mo–C bonding for sodium-ion capacitors. Nano Energy 2018, 47, 224–234. [Google Scholar] [CrossRef]
- Guo, H.; Ning, J.; Wang, B.; Feng, X.; Xia, M.; Wang, D.; Jia, Y.; Zhang, J.; Hao, Y. Sodium ion-intercalated nanoflower 1T–2H MoSe2-graphene nanocomposites as electrodes for all-solid-state supercapacitors. J. Alloys Compd. 2021, 853, 157116. [Google Scholar] [CrossRef]
- Xia, M.; Ning, J.; Wang, D.; Feng, X.; Wang, B.; Guo, H.; Zhang, J.; Hao, Y. Ammonia-assisted synthesis of gypsophila-like 1T-WSe2/graphene with enhanced potassium storage for all-solid-state supercapacitor. Chem. Eng. J. 2021, 405, 126611. [Google Scholar] [CrossRef]
- El-Mahalawy, S.H.; Evans, B.L. Temperature dependence of the electrical conductivity and hall coefficient in 2H-MoS2, MoSe2, WSe2, and MoTe2. Phys. Stat. Sol. 1977, 79, 713–722. [Google Scholar] [CrossRef]
- Lin, T.W.; Sadhasivam, T.; Wang, A.Y.; Chen, T.; Lin, J.; Shao, L. Ternary composite nanosheets with MoS2/WS2/Graphene heterostructures as high-performance cathode materials for supercapacitors. Chem Electro Chem 2018, 5, 1024–1031. [Google Scholar] [CrossRef]
- Wang, H.; Feng, H.; Li, J. Graphene and graphene-like layered transition metal dichalcogenides in energy conversion and storage. Small 2014, 10, 2165–2181. [Google Scholar] [CrossRef]
- Bissett, M.A.; Kinloch, I.A.; Dryfe, R.A.W. Characterization of MoS2-graphene composites for high-performance coin cell supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 17388–17398. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.M.; Bharath, G.; Hai, A.; Banat, F. Preparation of MoS2/graphene nanostructures and their supercapacitor and hydrogen evolution reaction (HER) performances. J. Phys. D 2019, 53, 065501. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, W.; Li, C.; He, L. First-principles study of nonmetal doped monolayer MoSe2 for tunable electronic and photocatalytic properties. Sci. Rep. 2017, 7, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Wang, W.; Li, C.; He, L. Tuning the magnetic properties of the monolayer MoSe2 by nonmetal doping: First-principles study. Solid State Commun. 2018, 281, 6–11. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, Y.; Guo, M.; Niu, C.; Lu, J.; Huang, B. Electronic and magnetic properties of perfect, vacancy-doped, and nonmetal adsorbed MoSe2, MoTe2 and WS 2 monolayers. Phys. Chem. Chem. Phys. 2011, 13, 15546–15553. [Google Scholar] [CrossRef]
- Sakthivel, M.; Ramaraj, S.; Chen, S.M.; Chen, T.W.; Ho, K.C. Transition-metal-doped molybdenum diselenides with defects and abundant active sites for efficient performances of enzymatic biofuel cell and supercapacitor applications. ACS Appl. Mater. Interfaces 2019, 11, 18483–18493. [Google Scholar] [CrossRef] [PubMed]
- Falola, B.D.; Fan, L.; Wiltowski, T.; Suni, I.I. Electrodeposition of Cu-doped MoS2 for charge storage in electrochemical supercapacitors. J. Electrochem. Soc. 2017, 164, D674–D679. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, J.; Zhu, Y.; Dumcenco, D.O.; Hong, J.; Mao, N.; Deng, S.; Chen, Y.; Yang, Y.; Jin, C.; et al. Two-dimensional molybdenum tungsten diselenide alloys: Photoluminescence, Raman scattering, and electrical transport. ACS Nano 2014, 8, 7130–7137. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.S.; Nagaraja, H.S. Effect of isoelectronic tungsten doping on molybdenum selenide nanostructures and their graphene hybrids for supercapacitors. Electrochim. Acta. 2019, 302, 459–471. [Google Scholar] [CrossRef]
- Ambrosi, A.; Sofer, Z.; Pumera, M. 2H→ 1T phase transition and hydrogen evolution activity of MoS2, MoSe2, WS 2 and WSe2 strongly depends on the MX 2 composition. Chem. Commun. 2015, 51, 8450–8453. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Mariappan, V.K.; Sahoo, S.; Manoharan, S.; Kim, S.J. A high efficacy self-charging MoSe2 solid-state supercapacitor using electrospun nanofibrous piezoelectric separator with Ionogel electrolyte. Adv. Mater. Interfaces 2018, 5, 1800055. [Google Scholar] [CrossRef]
- Qi, K.; Hou, R.; Zaman, S.; Qiu, Y.; Xia, B.Y.; Duan, H. Construction of metal–organic framework/conductive polymer hybrid for all-solid-state fabric supercapacitor. ACS Appl. Mater. Interfaces 2018, 10, 18021–18028. [Google Scholar] [CrossRef]
- Karade, S.S.; Dubal, D.P.; Sankapal, B.R. Decoration of ultrathin MoS2 nanoflakes over MWCNTs: Enhanced supercapacitive performance through electrode to symmetric all-solid-state device. ChemistrySelect 2017, 2, 10405–10412. [Google Scholar] [CrossRef]
- Yang, B.; Hao, C.; Wen, F.; Wang, B.; Mu, C.; Xiang, J.; Li, L.; Xu, B.; Zhao, Z.; Liu, Z.; et al. Flexible black-phosphorus nanoflake/carbon nanotube composite paper for high-performance all-solid-state supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 44478–44484. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Pazhamalai, P.; Veerasubramani, G.K.; Kim, S.J. Mechanically delaminated few layered MoS2 nanosheets based high performance wire type solid-state symmetric supercapacitors. J. Power Sources 2016, 321, 112–119. [Google Scholar] [CrossRef]
- Liu, F.; Zeng, L.; Chen, Y.; Zhang, R.; Yang, R.; Pang, J.; Ding, L.; Liu, H.; Zhou, W. Ni-Co-N hybrid porous nanosheets on graphene paper for flexible and editable asymmetric all-solid-state supercapacitors. Nano Energy 2019, 61, 18–26. [Google Scholar] [CrossRef]
- Hu, M.; Li, Z.; Li, G.; Hu, T.; Zhang, C.; Wang, X. All-solid-state flexible fiber-based MXene supercapacitors. Adv. Mater. Technol. 2017, 2, 1700143. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Ning, J.; Guo, H.; Xia, M.; Wang, B.; Feng, X.; Wang, D.; Zhang, J.; Hao, Y. Tungsten-Modulated Molybdenum Selenide/Graphene Heterostructure as an Advanced Electrode for All-Solid-State Supercapacitors. Nanomaterials 2021, 11, 1477. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11061477
Liu Q, Ning J, Guo H, Xia M, Wang B, Feng X, Wang D, Zhang J, Hao Y. Tungsten-Modulated Molybdenum Selenide/Graphene Heterostructure as an Advanced Electrode for All-Solid-State Supercapacitors. Nanomaterials. 2021; 11(6):1477. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11061477
Chicago/Turabian StyleLiu, Qixian, Jing Ning, Haibin Guo, Maoyang Xia, Boyu Wang, Xin Feng, Dong Wang, Jincheng Zhang, and Yue Hao. 2021. "Tungsten-Modulated Molybdenum Selenide/Graphene Heterostructure as an Advanced Electrode for All-Solid-State Supercapacitors" Nanomaterials 11, no. 6: 1477. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11061477