Pore Modification and Phosphorus Doping Effect on Phosphoric Acid-Activated Fe-N-C for Alkaline Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Ordered Mesoporous Silica Template, SBA-15
2.2. Synthesis of Fe-N-C Catalysts
2.3. Physical Characterization
2.4. Electrochemical Characterization
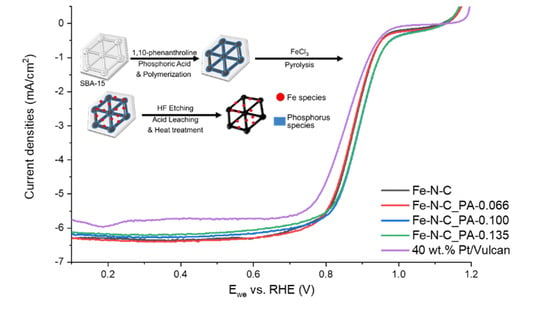
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panwar, N.; Kaushik, S.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Sheffield, J.W.; Sheffield, Ç. Assessment of Hydrogen Energy for Sustainable Development; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Dunn, S. Hydrogen futures: Toward a sustainable energy system. Int. J. Hydrogen Energy 2002, 27, 235–264. [Google Scholar] [CrossRef]
- Bailey, C.; Bain, A.; Birk, J.; Hainsselin, M.; Kamal, M.; Linden, H.; Lloyd, A.; Lynch, F.; Mackenzie, J.; Nahmias, D.; et al. The Green Hydrogen Report; National Renewable Energy Laboratory: Golden, CO, USA, 1995.
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Abe, J.; Popoola, A.; Ajenifuja, E.; Popoola, O. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Eftekhari, A.; Fang, B. Electrochemical hydrogen storage: Opportunities for fuel storage, batteries, fuel cells, and supercapacitors. Int. J. Hydrog. Energy 2017, 42, 25143–25165. [Google Scholar] [CrossRef]
- Wilson, A.; Kleen, G.; Papageorgopoulos, D. Fuel Cell System cost-2017 DOE Hydrogen and Fuel Cells Program. Record #17007, 2017. DOE Fuel Cell Technologies Office Record 17007: Fuel Cell System Cost–2017. Available online: energy.gov (accessed on 3 May 2021).
- Ren, X.; Lv, Q.; Liu, L.; Liu, B.; Wang, Y.; Liu, A.; Wu, G. Current progress of Pt and Pt-based electrocatalysts used for fuel cells. Sustain. Energy Fuels 2019, 4, 15–30. [Google Scholar] [CrossRef]
- Bhalothia, D.; Fan, Y.-J.; Lai, Y.-C.; Yang, Y.-T.; Yang, Y.-W.; Lee, C.-H.; Chen, T.-Y. Conformational Effects of Pt-Shells on Nanostructures and Corresponding Oxygen Reduction Reaction Activity of Au-Cluster-Decorated NiOx @Pt Nanocatalysts. Nanomaterials 2019, 9, 1003. [Google Scholar] [CrossRef] [Green Version]
- Karunagaran, R.; Tran, D.; Tung, T.T.; Shearer, C.; Losic, D. A Unique Synthesis of Macroporous N-Doped Carbon Composite Catalyst for Oxygen Reduction Reaction. Nanomaterials 2020, 11, 43. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, X.; Su, Y.-Q.; Wang, L.; Wang, H.; Dang, D.; Chi, B.; Liu, H.; Hensen, E.J.; Lou, X.W.; et al. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 2019, 366, 850–856. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, X.; Kang, Y.; Miao, L.; Xia, D.; Gan, L. Structurally Ordered Low-Pt Intermetallic Electrocatalysts toward Durably High Oxygen Reduction Reaction Activity. Adv. Funct. Mater. 2019, 29, 1902987. [Google Scholar] [CrossRef]
- Leteba, G.M.; Mitchell, D.R.G.; Levecque, P.B.J.; Lang, C.I. Solution-Grown Dendritic Pt-Based Ternary Nanostructures for Enhanced Oxygen Reduction Reaction Functionality. Nanomaterials 2018, 8, 462. [Google Scholar] [CrossRef] [Green Version]
- Menshchikov, V.; Alekseenko, A.; Guterman, V.; Nechitailov, A.; Glebova, N.; Tomasov, A.; Spiridonova, O.; Belenov, S.; Zelenina, N.; Safronenko, O. Effective Platinum-Copper Catalysts for Methanol Oxidation and Oxygen Reduction in Proton-Exchange Membrane Fuel Cell. Nanomaterials 2020, 10, 742. [Google Scholar] [CrossRef] [Green Version]
- Jia, Q.; Zhao, Z.; Cao, L.; Li, J.; Ghoshal, S.; Davies, V.; Stavitski, E.; Attenkofer, K.; Liu, Z.; Li, M.; et al. Roles of Mo Surface Dopants in Enhancing the ORR Performance of Octahedral PtNi Nanoparticles. Nano Lett. 2018, 18, 798–804. [Google Scholar] [CrossRef]
- Beermann, V.; Gocyla, M.; Kühl, S.; Padgett, E.; Schmies, H.; Goerlin, M.; Erini, N.; Shviro, M.; Heggen, M.; Dunin-Borkowski, R.E.; et al. Tuning the Electrocatalytic Oxygen Reduction Reaction Activity and Stability of Shape-Controlled Pt–Ni Nanoparticles by Thermal Annealing − Elucidating the Surface Atomic Structural and Compositional Changes. J. Am. Chem. Soc. 2017, 139, 16536–16547. [Google Scholar] [CrossRef]
- Jung, D.; Beak, S.; Nahm, K.S.; Kim, P. Enhancement of oxygen reduction activity by sequential impregnation of Pt and Pd on carbon support. Korean J. Chem. Eng. 2010, 27, 1689–1694. [Google Scholar] [CrossRef]
- Kang, Y.S.; Choi, D.; Park, H.-Y.; Yoo, S.J. Tuning the surface structure of PtCo nanocatalysts with high activity and stability toward oxygen reduction. J. Ind. Eng. Chem. 2019, 78, 448–454. [Google Scholar] [CrossRef]
- Delaporte, N.; Rivard, E.; Natarajan, S.K.; Benard, P.; Trudeau, M.L.; Zaghib, K. Synthesis and Performance of MOF-Based Non-Noble Metal Catalysts for the Oxygen Reduction Reaction in Proton-Exchange Membrane Fuel Cells: A Review. Nanomaterials 2020, 10, 1947. [Google Scholar] [CrossRef]
- Kim, S.; Kato, S.; Ishizaki, T.; Li, O.L.; Kang, J. Transition Metal (Fe, Co, Ni) Nanoparticles on Selective Amino-N-Doped Carbon as High-Performance Oxygen Reduction Reaction Electrocatalyst. Nanomaterials 2019, 9, 742. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhou, W.; Wang, H.; Xie, L.; Liang, Y.; Wei, F.; Idrobo, J.C.; Pennycook, S.J.; Dai, H. An oxygen reduction electrocatalyst based on carbon nanotube–graphene complexes. Nat. Nanotechnol. 2012, 7, 394–400. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [Green Version]
- Sathiskumar, C.; Ramakrishnan, S.; Vinothkannan, M.; Kim, A.R.; Karthikeyan, S.; Yoo, D.J. Nitrogen-Doped Porous Carbon Derived from Biomass Used as Trifunctional Electrocatalyst toward Oxygen Reduction, Oxygen Evolution and Hydrogen Evolution Reactions. Nanomaterials 2019, 10, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Dai, L. Heteroatom-Doped Graphitic Carbon Catalysts for Efficient Electrocatalysis of Oxygen Reduction Reaction. ACS Catal. 2015, 5, 7244–7253. [Google Scholar] [CrossRef]
- Lv, Q.; Si, W.; He, J.; Sun, L.; Zhang, C.; Wang, N.; Yang, Z.; Li, X.; Wang, X.; Deng, W.; et al. Selectively nitrogen-doped carbon materials as superior metal-free catalysts for oxygen reduction. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Wie, J.; Kim, J. Synergistic interaction of P and N co-doping EDTA with controllable active EDTA-cobalt sites as efficient electrocatalyst for oxygen reduction reaction. J. Ind. Eng. Chem. 2020, 83, 252–259. [Google Scholar] [CrossRef]
- Chen, Z.; Higgins, D.; Yu, A.; Zhang, L.; Zhang, J. A review on non-precious metal electrocatalysts for PEM fuel cells. Energy Environ. Sci. 2011, 4, 3167–3192. [Google Scholar] [CrossRef]
- Othman, R.; Dicks, A.L.; Zhu, Z. Non precious metal catalysts for the PEM fuel cell cathode. Int. J. Hydrog. Energy 2012, 37, 357–372. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Han, S.-B.; Kwak, D.-H.; Song, J.; Park, K.-W. Doped porous carbon nanostructure materials as non-precious metal catalysts for oxygen reduction reaction in alkaline and acid media. J. Ind. Eng. Chem. 2019, 80, 171–181. [Google Scholar] [CrossRef]
- Roh, C.-W.; Lee, H. Fe/N/C catalysts synthesized using graphene aerogel for electrocatalytic oxygen reduction reaction in an acidic condition. Korean J. Chem. Eng. 2016, 33, 2582–2588. [Google Scholar] [CrossRef]
- He, Y.; Guo, H.; Hwang, S.; Yang, X.; He, Z.; Braaten, J.; Karakalos, S.; Shan, W.; Wang, M.; Zhou, H.; et al. Single Cobalt Sites Dispersed in Hierarchical-ly Porous Nanofiber Networks for Durable and High-Power PGM-Free Cathodes in Fuel Cells. Adv. Mater. 2020, 32, 2003577. [Google Scholar] [CrossRef]
- Martinez, U.; Babu, S.K.; Holby, E.F.; Chung, H.T.; Yin, X.; Zelenay, P. Progress in the Development of Fe-Based PGM-Free Electrocatalysts for the Oxygen Reduction Reaction. Adv. Mater. 2019, 31, e1806545. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.; Chung, D.Y.; Yoo, J.M.; Lee, H.S.; Kim, M.J.; Mun, B.S.; Kwon, S.G.; Sung, Y.-E.; Hyeon, T. Design Principle of Fe–N–C Electrocatalysts: How to Optimize Multimodal Porous Structures? J. Am. Chem. Soc. 2019, 141, 2035–2045. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Lin, H.; Gupta, S.; Wang, C.; Tao, Z.; Fu, H.; Wang, T.; Zheng, J.; Wu, G.; et al. Directly converting Fe-doped metal–organic frameworks into highly active and stable Fe-N-C catalysts for oxygen reduction in acid. Nano Energy 2016, 25, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Mustain, W.E.; Chatenet, M.; Page, M.; Kim, Y.S. Durability challenges of anion exchange membrane fuel cells. Energy Environ. Sci. 2020, 13, 2805–2838. [Google Scholar] [CrossRef]
- Cha, M.S.; Park, J.E.; Kim, S.; Han, S.-H.; Shin, S.-H.; Yang, S.H.; Kim, T.-H.; Yu, D.M.; So, S.; Hong, Y.T.; et al. Poly(carbazole)-based anion-conducting materials with high performance and durability for energy conversion devices. Energy Environ. Sci. 2020, 13, 3633–3645. [Google Scholar] [CrossRef]
- Shao, Y.; Dodelet, J.; Wu, G.; Zelenay, P. PGM-Free Cathode Catalysts for PEM Fuel Cells: A Mini-Review on Stability Challenges. Adv. Mater. 2019, 31, e1807615. [Google Scholar] [CrossRef]
- Osmieri, L.; Cullen, D.A.; Chung, H.T.; Ahluwalia, R.K.; Neyerlin, K. Durability evaluation of a Fe–N–C catalyst in polymer electrolyte fuel cell environment via accelerated stress tests. Nano Energy 2020, 78, 105209. [Google Scholar] [CrossRef]
- Yuan, K.; Lützenkirchen-Hecht, D.F.; Li, L.; Shuai, L.; Li, Y.; Cao, R.; Qiu, M.; Zhuang, X.; Leung, M.K.H.; Chen, Y.; et al. Boosting Oxygen Reduction of Single Iron Active Sites via Geometric and Electronic Engineering: Nitrogen and Phosphorus Dual Coordination. J. Am. Chem. Soc. 2020, 142, 2404–2412. [Google Scholar] [CrossRef]
- Pan, F.; Li, B.; Sarnello, E.; Hwang, S.; Gang, Y.; Feng, X.; Xiang, X.; Adli, N.M.; Li, T.; Su, D.; et al. Boosting CO2 reduction on Fe-N-C with sulfur incorporation: Synergistic electronic and structural engineering. Nano Energy 2020, 68, 104384. [Google Scholar] [CrossRef]
- Bai, L.; Liu, J.; Jin, C.; Zhang, J.; Wang, F. Heteroatom-doped carbon interpenetrating networks: A signpost to achieve the best performance of non-PGM catalysts for fuel cells. J. Mater. Chem. A 2020, 8, 18767–18777. [Google Scholar] [CrossRef]
- Proietti, E.; Jaouen, F.; Lefèvre, M.; Larouche, N.; Tian, J.; Herranz, J.; Dodelet, J.-P. Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells. Nat. Commun. 2011, 2, 416. [Google Scholar] [CrossRef]
- Lai, Q.; Zhao, Y.; Liang, Y.; He, J.; Chen, J. In Situ Confinement Pyrolysis Transformation of ZIF-8 to Nitrogen-Enriched Meso-Microporous Carbon Frameworks for Oxygen Reduction. Adv. Funct. Mater. 2016, 26, 8334–8344. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Cullen, D.A.; Hwang, S.; Wang, M.; Li, B.; Liu, K.; Karakalos, S.; Lucero, M.; Zhang, H.; et al. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat. Catal. 2018, 1, 935–945. [Google Scholar] [CrossRef]
- Sa, Y.J.; Seo, D.-J.; Dongwoo, K.; Lim, J.T.; Cheon, J.Y.; Yang, S.Y.; Lee, J.M.; Kang, D.; Shin, T.J.; Shin, H.S.; et al. A General Approach to Preferential Formation of Active Fe–Nx Sites in Fe–N/C Electrocatalysts for Efficient Oxygen Reduction Reaction. J. Am. Chem. Soc. 2016, 138, 15046–15056. [Google Scholar] [CrossRef]
- Shen, H.; Gracia-Espino, E.; Ma, J.; Tang, H.; Mamat, X.; Wagberg, T.; Hu, G.; Guo, S. Atomically FeN2 moieties dispersed on mesoporous carbon: A new atomic catalyst for efficient oxygen reduction catalysis. Nano Energy 2017, 35, 9–16. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, S.; Zhao, Y.; Zhu, L.; Chen, S.; Wang, X.; Wu, Q.; Ma, J.; Ma, Y.; Hu, Z. Boron-Doped Carbon Nanotubes as Metal-Free Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2011, 50, 7132–7135. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Bae, E.J.; Yu, J.-S. Fe–P: A New Class of Electroactive Catalyst for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2015, 137, 3165–3168. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ji, S.; Chen, C.; Peng, Q.; Wang, D.; Li, Y. Single-Atom Catalysts: Synthetic Strategies and Electrochemical Applications. Joule 2018, 2, 1242–1264. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Yuan, P.; Zhang, J.; Jin, P.; Sun, H.; Lei, K.; Pang, X.; Xu, Q.; Cheng, F. Atomic-scaled cobalt encapsulated in P,N-doped carbon sheaths over carbon nanotubes for enhanced oxygen reduction electrocatalysis under acidic and alkaline media. Chem. Commun. 2017, 53, 9862–9865. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Xie, S.; Li, L.; Fan, J.; Li, Q.; Min, Y.; Xu, Q. Highly accessible sites of Fe-N on biomass-derived N, P co-doped hierarchical porous carbon for oxygen reduction reaction. J. Nanopart. Res. 2021, 23, 1–14. [Google Scholar] [CrossRef]
- Deng, J.; Chen, S.; Zhou, Q.; Nie, Y.; Li, J.; Wu, R.; Wang, Q.; Wei, Z. Phytic acid-assisted self-templating synthesis of N-P-Fe-tridoped hierarchical porous carbon for efficient oxygen reduction reaction. J. Power Sources 2020, 451, 227808. [Google Scholar] [CrossRef]
- Jin, X.; Lee, C.H.; Kim, J.H.; You, D.J.; Pak, C.; Shon, J.K.; Kim, J.M. Systematically Controlled Pore System of Ordered Mesoporous Carbons Using Phosphoric Acid as the In situ Generated Catalysts for Carbonization and Activation. Bull. Korean Chem. Soc. 2015, 36, 2062–2067. [Google Scholar] [CrossRef]
- Joo, S.H.; Pak, C.; You, D.J.; Lee, S.-A.; Lee, H.I.; Kim, J.M.; Chang, H.; Seung, D. Ordered mesoporous carbons (OMC) as supports of electrocatalysts for direct methanol fuel cells (DMFC): Effect of carbon precursors of OMC on DMFC performances. Electrochim. Acta 2006, 52, 1618–1626. [Google Scholar] [CrossRef]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef] [Green Version]
- Hoekstra, J.; Beale, A.; Soulimani, F.; Versluijs-Helder, M.; van de Kleut, D.; Koelewijn, J.M.; Geus, J.W.; Jenneskens, L.W. The effect of iron catalyzed graphitization on the textural properties of carbonized cellulose: Magnetically separable graphitic carbon bodies for catalysis and remediation. Carbon 2016, 107, 248–260. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.G.; Pak, C. Electrolyte accessibility of non-precious-metal catalysts with different spherical particle sizes under alkaline conditions for oxygen reduction reaction. J. Energy Chem. 2021, 52, 326–331. [Google Scholar] [CrossRef]
- Lee, H.I.; Kim, J.H.; You, D.J.; Lee, J.E.; Ahn, W.S.; Pak, C.; Joo, S.H.; Chang, H.; Seung, D. Rational Synthesis Pathway for Ordered Mesoporous Carbon with Controllable 30- to 100-Angstrom Pores. Adv. Mater. 2008, 20, 757–762. [Google Scholar] [CrossRef]
- Yin, X.; Chung, H.T.; Martinez, U.; Lin, L.; Artyushkova, K.; Zelenay, P. PGM-Free ORR Catalysts Designed by Templating PANI-Type Polymers Containing Functional Groups with High Affinity to Iron. J. Electrochem. Soc. 2019, 166, F3240–F3245. [Google Scholar] [CrossRef]
- Divya, K.; Chandran, A.; Reethu, V.; Mathew, S. Enhanced photocatalytic performance of RGO/Ag nanocomposites produced via a facile microwave irradiation for the degradation of Rhodamine B in aqueous solution. Appl. Surf. Sci. 2018, 444, 811–818. [Google Scholar] [CrossRef]
- Ba, H.; Luo, J.; Liu, Y.; Duong-Viet, C.; Tuci, G.; Giambastiani, G.; Nhut, J.-M.; Nguyen-Dinh, L.; Ersen, O.; Su, D.S.; et al. Macroscopically shaped monolith of nanodiamonds @ nitrogen-enriched mesoporous carbon decorated SiC as a superior metal-free catalyst for the styrene production. Appl. Catal. B Environ. 2017, 200, 343–350. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Wang, R.; Hong, M. Structural Evolution from Metal–Organic Framework to Hybrids of Nitrogen-Doped Porous Carbon and Carbon Nanotubes for Enhanced Oxygen Reduction Activity. Chem. Mater. 2015, 27, 7610–7618. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, J.; Pan, Z.; Wang, X.; Liu, S.; Ding, S.; Lang, L. An efficient metal-free catalyst derived from waste lotus seedpod for oxygen reduction reaction. J. Porous Mater. 2020, 27, 637–646. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, H.; Li, J.; Yang, X. Achieving a high-performance P/C anode through P-O-C bond for sodium ion batteries. Ionics 2020, 26, 3377–3385. [Google Scholar] [CrossRef]
- Wang, Q.; Hou, M.; Huang, Y.; Li, J.; Zhou, X.; Ma, G.; Ren, S. One-pot synthesis of NiCoP/CNTs composites for lithium ion batteries and hydrogen evolution reaction. Ionics 2019, 26, 1771–1778. [Google Scholar] [CrossRef]
- Adli, N.M.; Shan, W.; Hwang, S.; Samarakoon, W.; Karakalos, S.; Li, Y.; Cullen, D.A.; Su, D.; Feng, Z.; Wang, G.; et al. Engineering Atomically Dispersed FeN4 Active Sites for CO2 Electroreduction. Angew. Chem. 2021, 133, 1035–1045. [Google Scholar] [CrossRef]
- Zheng, L.; Yu, S.; Lu, X.; Fan, W.; Chi, B.; Ye, Y.; Shi, X.; Zeng, J.; Li, X.; Liao, S. Two-Dimensional Bimetallic Zn/Fe-Metal-Organic Framework (MOF)-Derived Porous Carbon Nanosheets with a High Density of Single/Paired Fe Atoms as High-Performance Oxygen Reduction Catalysts. ACS Appl. Mater. Interfaces 2020, 12, 13878–13887. [Google Scholar] [CrossRef]
- Sun, X.; Wei, P.; Gu, S.; Zhang, J.; Jiang, Z.; Wan, J.; Chen, Z.; Huang, L.; Xu, Y.; Fang, C.; et al. Atomic-Level Fe-N-C Coupled with Fe3C-Fe Nanocomposites in Carbon Matrixes as High-Efficiency Bifunctional Oxygen Catalysts. Small 2019, 16, 1906057. [Google Scholar]
- Dai, C.; Fan, F.; Lv, M.; Pan, L.; Liang, S.; Wang, J.; Shen, H.; Thomas, T.; Yang, M. Communication—Fe/FeNi3 Embedded in Nitrogen-Doped Carbon Nanotubes as Bifunctional Oxygen Electrocatalysts. J. Electrochem. Soc. 2020, 167, 146504. [Google Scholar] [CrossRef]
- Li, H.; Du, K.; Xiang, C.; An, P.; Shu, X.; Dang, Y.; Wu, C.; Wang, J.; Du, W.; Zhang, J.; et al. Controlled chelation between tannic acid and Fe precursors to obtain N, S co-doped carbon with high density Fe-single atom-nanoclusters for highly efficient oxygen reduction reaction in Zn–air batteries. J. Mater. Chem. A 2020, 8, 17136–17149. [Google Scholar] [CrossRef]
- Shao, C.; Zhuang, S.; Zhang, H.; Jiang, Q.; Xu, X.; Ye, J.; Li, B.; Wang, X. Enhancement of Mass Transport for Oxygen Reduction Reaction Using Petal-Like Porous Fe-NC Nanosheet. Small 2021, 17, e2006178. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, M.; Zuo, J.; Wang, J.; Liu, Z.; Wang, L.; Liu, B.; Wu, G.; Zhang, H.; Yang, H. Fe-Doped Metal-Organic Frameworks-Derived Electrocatalysts for Oxygen Reduction Reaction in Alkaline Media. J. Electrochem. Soc. 2018, 165, F1278–F1285. [Google Scholar] [CrossRef]
- Jiang, T.; Luan, W.; Ren, Y.; Fan, C.; Feng, Q.; Turyanska, L. Synergistic heat treatment derived hollow-mesoporous-microporous Fe–N–C-SHT electrocatalyst for oxygen reduction reaction. Microporous Mesoporous Mater. 2020, 305, 110382. [Google Scholar] [CrossRef]
- Li, Q.; Liu, H.; Zhang, L.-C.; Chen, H.; Zhu, H.; Wu, Y.; Xu, M.; Bao, S.-J. Highly efficient Fe-N-C oxygen reduction electrocatalyst engineered by sintering atmosphere. J. Power Sources 2020, 449, 227497. [Google Scholar] [CrossRef]
- Yin, S.; Li, G.; Qu, X.-M.; Zhang, J.; Shen, L.; Li, Y.; Wang, C.; Yu, Z.; Lu, B.-A.; Xu, B.; et al. Self-Template Synthesis of Atomically Dispersed Fe/N-Codoped Nanocarbon as Efficient Bifunctional Alkaline Oxygen Electrocatalyst. ACS Appl. Energy Mater. 2019, 3, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Hossen, M.; Artyushkova, K.; Atanassov, P.; Serov, A. Synthesis and characterization of high performing Fe-N-C catalyst for oxygen reduction reaction (ORR) in Alkaline Exchange Membrane Fuel Cells. J. Power Sources 2018, 375, 214–221. [Google Scholar] [CrossRef]
- Chena, Y.; Shia, Z.; Wanga, Z.; Wanga, C.; Fenga, J.; Panga, B.; Suna, Q.; Yua, L.; Dongab, L. Yolk-shell Fe@FeNx nanoparticles decorated N-doped mesoporous carbon as highly active electrocatalyst for oxygen reduction reactions. J. Alloys Compd. 2020, 829, 154558. [Google Scholar] [CrossRef]










| SBET (m2/g) | Vp (cm3/g) | Vp,micro (cm3/g) | Vp,meso (cm3/g) | |
|---|---|---|---|---|
| SBA-15 | 680 | 0.96 | 0.11 | 0.85 |
| Fe-N-C | 1070 | 0.83 | 0.34 | 0.49 |
| Fe-N-C_PA-0.066 | 1050 | 0.60 | 0.31 | 0.29 |
| Fe-N-C_PA-0.100 | 1110 | 0.68 | 0.30 | 0.38 |
| Fe-N-C_PA-0.135 | 1200 | 0.69 | 0.34 | 0.35 |
| at% | Fe | P | N | C |
|---|---|---|---|---|
| Fe-N-C | <0.1 | − | 3.26 | 94.44 |
| Fe-N-C_PA-0.066 | <0.1 | 0.18 | 3.31 | 93.32 |
| Fe-N-C_PA-0.100 | <0.1 | 0.29 | 3.15 | 93.27 |
| Fe-N-C_PA-0.135 | <0.1 | 0.56 | 3.20 | 92.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.G.; Han, S.; Pak, C. Pore Modification and Phosphorus Doping Effect on Phosphoric Acid-Activated Fe-N-C for Alkaline Oxygen Reduction Reaction. Nanomaterials 2021, 11, 1519. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11061519
Kim JG, Han S, Pak C. Pore Modification and Phosphorus Doping Effect on Phosphoric Acid-Activated Fe-N-C for Alkaline Oxygen Reduction Reaction. Nanomaterials. 2021; 11(6):1519. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11061519
Chicago/Turabian StyleKim, Jong Gyeong, Sunghoon Han, and Chanho Pak. 2021. "Pore Modification and Phosphorus Doping Effect on Phosphoric Acid-Activated Fe-N-C for Alkaline Oxygen Reduction Reaction" Nanomaterials 11, no. 6: 1519. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11061519