Microwave-Assisted Rapid Synthesis of NH4V4O10 Layered Oxide: A High Energy Cathode for Aqueous Rechargeable Zinc Ion Batteries
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Structural and Morphological Characterization
2.2. Electrochemical Characterization
3. Results and Discussion
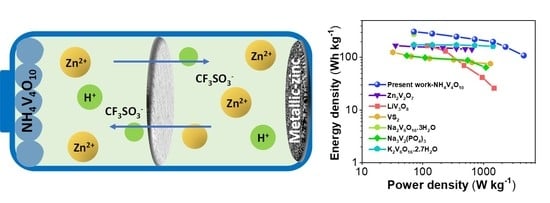
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, X.; Liu, C.; Neale, Z.G.; Yang, J.; Cao, G. Active Materials for Aqueous Zinc Ion Batteries: Synthesis, Crystal Structure, Morphology, and Electrochemistry. Chem. Rev. 2020, 120, 7795–7866. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Liu, F.; Luo, Z.; Fang, G.; Zhou, J.; Pan, A.; Liang, S. Pilotaxitic Na1.1V3O7.9 nanoribbons/graphene as high-performance sodium ion battery and aqueous zinc ion battery cathode. Energy Storage Mater. 2018, 13, 168–174. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, F.; Liu, Y.; Zhao, Q.; Lei, K.; Chen, C.; Liu, X.; Chen, J. Cation-Deficient Spinel ZnMn2O4 Cathode in Zn(CF3SO3)2 Electrolyte for Rechargeable Aqueous Zn-Ion Battery. J. Am. Chem. Soc. 2016, 138, 12894. [Google Scholar] [CrossRef]
- Zhao, H.B.; Hu, C.J.; Cheng, H.W.; Fang, J.H.; Xie, Y.P.; Fang, W.Y.; Doan, T.N.L.; Hoang, T.K.A.; Xu, J.Q.; Chen, P. Novel Rechargeable M3V2(PO4)3//Zinc (M = Li, Na) Hybrid Aqueous Batteries with Excellent Cycling Performance. Sci. Rep. 2016, 6, 25809. [Google Scholar] [CrossRef] [Green Version]
- Hou, Z.; Zhang, X.; Li, X.; Zhu, Y.; Liang, J.; Qian, Y. Surfactant widens the electrochemical window of an aqueous electrolyte for better rechargeable aqueous sodium/zinc battery. J. Mater. Chem. A 2017, 5, 730–738. [Google Scholar] [CrossRef]
- Soundharrajan, V.; Sambandam, B.; Kim, S.; Mathew, V.; Jo, J.; Kim, S.; Lee, J.; Islam, S.; Kim, K.; Sun, Y.-K.; et al. Aqueous Magnesium Zinc Hybrid Battery: An Advanced High Voltage and High Energy MgMn2O4 Cathode. ACS Energy Lett. 2018, 3, 1998–2004. [Google Scholar] [CrossRef]
- Mathew, V.; Sambandam, B.; Kim, S.; Kim, S.; Park, S.; Lee, S.; Alfaruqi, M.H.; Soundharrajan, V.; Islam, S.; Putro, D.Y.; et al. Manganese and Vanadium Oxide Cathodes for Aqueous Rechargeable Zinc-Ion Batteries: A Focused View on Performance, Mechanism, and Developments. ACS Energy Lett. 2020, 5, 2376–2400. [Google Scholar] [CrossRef]
- Soundharrajan, V.; Sambandam, B.; Kim, S.; Islam, S.; Jo, J.; Kim, S.; Mathew, V.; Sun, Y.; Kim, J. The dominant role of Mn2+ additive on the electrochemical reaction in ZnMn2O4 cathode for aqueous zinc-ion batteries. Energy Storage Mater. 2020, 28, 407–417. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Zhou, X.; Liu, Z. Towards High-Voltage Aqueous Metal-Ion Batteries Beyond 1.5 V: The Zinc/Zinc Hexacyanoferrate System. Adv. Energy Mater. 2015, 5, 1400930. [Google Scholar] [CrossRef]
- Chamoun, M.; Brant, W.R.; Tai, C.-W.; Karlsson, G.; Noréus, D. Rechargeability of aqueous sulfate Zn/MnO2 batteries enhanced by accessible Mn2+ ions. Energy Storage Mater. 2018, 15, 351–360. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Gim, J.; Kim, S.; Song, J.; Jo, J.; Kim, S.; Mathew, V.; Kim, J. Enhanced reversible divalent zinc storage in a structurally stable α-MnO2 nanorod electrode. J. Power Sources 2015, 288, 320–327. [Google Scholar] [CrossRef]
- Bischoff, C.F.; Fitz, O.S.; Burns, J.; Bauer, M.; Gentischer, H.; Birke, K.P.; Henning, H.-M.; Biro, D. Revealing the Local pH Value Changes of Acidic Aqueous Zinc Ion Batteries with a Manganese Dioxide Electrode during Cycling. J. Electrochem. Soc. 2020, 167, 020545. [Google Scholar] [CrossRef]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Jiang, B.; Xu, C.; Wu, C.; Dong, L.; Li, J.; Kang, F. Manganese Sesquioxide as Cathode Material for Multivalent Zinc Ion Battery with High Capacity and Long Cycle Life. Electrochim. Acta 2017, 229, 422–428. [Google Scholar] [CrossRef]
- Islam, S.; Alfaruqi, M.H.; Mathew, V.; Song, J.; Kim, S.; Kim, S.; Jo, J.; Baboo, J.P.; Pham, D.T.; Putro, D.Y.; et al. Facile synthesis and the exploration of the zinc storage mechanism of β-MnO2 nanorods with exposed (101) planes as a novel cathode material for high performance eco-friendly zinc-ion batteries. J. Mater. Chem. A 2017, 5, 23299–23309. [Google Scholar] [CrossRef]
- Sun, W.; Wang, F.; Hou, S.; Yang, C.; Fan, X.; Ma, Z.; Gao, T.; Han, F.; Hu, R.; Zhu, M.; et al. Zn/MnO2 Battery Chemistry With H+ and Zn2+ Coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, J.; Li, J.; Cao, L.; Xu, Z.; Wu, J.; Cao, S.; Hu, H. Structure-controlled synthesis and electrochemical properties of NH4V3O8 as cathode material for Lithium ion batteries. Electrochim. Acta 2016, 212, 217–224. [Google Scholar] [CrossRef]
- Soundharrajan, V.; Sambandam, B.; Song, J.; Kim, S.; Jo, J.; Duong, P.T.; Kim, S.; Mathew, V.; Kim, J. Facile green synthesis of a Co3V2O8 nanoparticle electrode for high energy lithium-ion battery applications. J. Colloid Interface Sci. 2017, 501, 133–141. [Google Scholar] [CrossRef]
- Soundharrajan, V.; Sambandam, B.; Song, J.; Kim, S.; Jo, J.; Pham, D.T.; Kim, S.; Mathew, V.; Kim, J. Bitter gourd-shaped Ni3V2O8 anode developed by a one-pot metal-organic framework-combustion technique for advanced Li-ion batteries. Ceram. Int. 2017, 43, 13224–13232. [Google Scholar] [CrossRef]
- Soundharrajan, V.; Sambandam, B.; Song, J.; Kim, S.; Jo, J.; Kim, S.; Lee, S.; Mathew, V.; Kim, J. Co3V2O8 Sponge Network Morphology Derived from Metal–Organic Framework as an Excellent Lithium Storage Anode Material. ACS Appl. Mater. Interfaces 2016, 8, 8546–8553. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, G.S.; Ottmann, A.; Ehrstein, B.; Tyutyunnik, A.P.; Zhu, Q.; Lu, S.; Voronin, V.I.; Enyashin, A.N.; Klingeler, R. A new polymorph of NH4V3O7: Synthesis, structure, magnetic and electrochemical properties. Solid State Sci. 2016, 61, 225–231. [Google Scholar] [CrossRef]
- Sambandam, B.; Soundharrajan, V.; Kim, S.; Alfaruqi, M.H.; Jo, J.; Kim, S.; Mathew, V.; Sun, Y.-K.; Kim, J. Aqueous rechargeable Zn-ion battery: An imperishable and high-energy Zn2V2O7 nanowire cathode through intercalation regulation. J. Mater. Chem. A 2018, 6, 3850–3856. [Google Scholar] [CrossRef]
- Sambandam, B.; Kim, S.; Pham, D.T.; Mathew, V.; Lee, J.; Lee, S.; Soundharrajan, V.; Kim, S.; Alfaruqi, M.H.; Hwang, J.-Y.; et al. Hyper oxidized V6O13+x·nH2O layered cathode for aqueous rechargeable Zn battery: Effect on dual carriers transportation and parasitic reactions. Energy Storage Mater. 2021, 35, 47–61. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X. Review of vanadium-based electrode materials for rechargeable aqueous zinc ion batteries. J. Energy Chem. 2021, 56, 223–237. [Google Scholar] [CrossRef]
- Kundu, D.; Adams, B.D.; Duffort, V.; Vajargah, S.H.; Nazar, L.F. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 2016, 1, 16119. [Google Scholar] [CrossRef]
- He, P.; Quan, Y.; Xu, X.; Yan, M.; Yang, W.; An, Q.; He, L.; Mai, L.Q. High-Performance Aqueous Zinc-Ion Battery Based on Layered H2V3O8 Nanowire Cathode. Small 2017, 13, 1702551. [Google Scholar] [CrossRef]
- Xia, C.; Guo, J.; Li, P.; Zhang, X.; Alshareef, H.N. Highly Stable Aqueous Zinc-Ion Storage Using a Layered Calcium Vanadium Oxide Bronze Cathode. Angew. Chem. Int. Ed. 2018, 57, 3943–3948. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Mathew, V.; Song, J.; Kim, S.; Islam, S.; Pham, D.T.; Jo, J.; Kim, S.; Baboo, J.P.; Xiu, Z.; et al. Electrochemical Zinc Intercalation in Lithium Vanadium Oxide: A High-Capacity Zinc-Ion Battery Cathode. Chem. Mater. 2017, 29, 1684–1694. [Google Scholar] [CrossRef]
- Li, G.; Yang, Z.; Jiang, Y.; Jin, C.; Huang, W.; Ding, X.; Huang, Y. Towards Polyvalent Ion Batteries: A Zinc-Ion Battery Based on NASICON Structured Na3V2(PO4)3. Nano Energy 2016, 25, 211. [Google Scholar] [CrossRef]
- He, P.; Yan, M.; Zhang, G.; Sun, R.; Chen, L.; An, Q.; Mai, L. Layered VS2 Nanosheet-Based Aqueous Zn Ion Battery Cathode. Adv. Energy Mater. 2017, 7, 1601920. [Google Scholar] [CrossRef]
- He, P.; Zhang, G.; Liao, X.; Yan, M.; Xu, X.; An, Q.; Liu, J.; Mai, L.Q. Sodium Ion Stabilized Vanadium Oxide Nanowire Cathode for High-Performance Zinc-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702463. [Google Scholar] [CrossRef]
- Tang, B.; Zhou, J.; Fang, G.; Liu, F.; Zhu, C.; Wang, C.; Pan, A.; Liang, S. Engineering the interplanar spacing of ammonium vanadates as a high-performance aqueous zinc-ion battery cathode. J. Mater. Chem. A 2019, 7, 940–945. [Google Scholar] [CrossRef]
- Li, Q.; Rui, X.; Chen, D.; Feng, Y.; Xiao, N.; Gan, L.; Zhang, Q.; Yu, Y.; Huang, S. A High-Capacity Ammonium Vanadate Cathode for Zinc-Ion Battery. Nano-Micro Lett. 2020, 12, 67. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Wei, T.; Wang, C. Self-Healing Lamellar Structure Boosts Highly Stable Zinc-Storage Property of Bilayered Vanadium Oxides. ACS Appl. Mater. Interfaces 2018, 10, 35079–35089. [Google Scholar] [CrossRef]
- Qiu, N.; Chen, H.; Yang, Z.; Zhu, Y.; Liu, W.; Wang, Y. Porous hydrated ammonium vanadate as a novel cathode for aqueous rechargeable Zn-ion batteries. Chem. Commun. 2020, 56, 3785–3788. [Google Scholar] [CrossRef]
- Bilecka, I.; Niederberger, M. Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale 2010, 2, 1358–1374. [Google Scholar] [CrossRef]
- Balaji, S.; Mutharasu, D.; Sankara Subramanian, N.; Ramanathan, K. A review on microwave synthesis of electrode materials for lithium-ion batteries. Ionics 2009, 15, 765. [Google Scholar] [CrossRef]
- Haruna, A.B.; Ozoemena, K.I. Effects of microwave irradiation on the electrochemical performance of manganese-based cathode materials for lithium-ion batteries. Curr. Opin. Electrochem. 2019, 18, 16–23. [Google Scholar] [CrossRef]
- Sambandam, B.; Soundharrajan, V.; Kim, S.; Alfaruqi, M.H.; Jo, J.; Kim, S.; Mathew, V.; Sun, Y.; Kim, J. K2V6O16·2.7H2O nanorod cathode: An advanced intercalation system for high energy aqueous rechargeable Zn-ion batteries. J. Mater. Chem. A 2018, 6, 15530–15539. [Google Scholar] [CrossRef]
- Liu, W.; Dong, L.; Jiang, B.; Huang, Y.; Wang, X.; Xu, C.; Kang, Z.; Mou, J.; Kang, F. Layered vanadium oxides with proton and zinc ion insertion for zinc ion batteries. Electrochim. Acta 2019, 320, 134565. [Google Scholar] [CrossRef]
- Wan, F.; Zhang, L.; Dai, X.; Wang, X.; Niu, Z.; Chen, J. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers. Nat. Commun. 2018, 9, 1656. [Google Scholar] [CrossRef] [Green Version]
- Esparcia, E.A.; Chae, M.S.; Ocon, J.D.; Hong, S.-T. Ammonium Vanadium Bronze (NH4V4O10) as a High-Capacity Cathode Material for Nonaqueous Magnesium-Ion Batteries. Chem. Mater. 2018, 30, 3690–3696. [Google Scholar] [CrossRef]
- Soundharrajan, V.; Sambandam, B.; Kim, S.; Alfaruqi, M.H.; Putro, D.Y.; Jo, J.; Kim, S.; Mathew, V.; Sun, Y.-K.; Kim, J. Na2V6O16·3H2O Barnesite Nanorod: An Open Door to Display a Stable and High Energy for Aqueous Rechargeable Zn-Ion Batteries as Cathodes. Nano Lett. 2018, 18, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Soundharrajan, V.; Sambandam, B.; Alfaruqi, M.H.; Kim, S.; Jo, J.; Kim, S.; Mathew, V.; Sun, Y.; Kim, J. Na2.3Cu1.1Mn2O7−δ nanoflakes as enhanced cathode materials for high-energy sodium-ion batteries achieved by a rapid pyrosynthesis approach. J. Mater. Chem. A 2020, 8, 770–778. [Google Scholar] [CrossRef]
- Soundharrajan, V.; Alfaruqi, M.H.; Lee, S.; Sambandam, B.; Kim, S.; Kim, S.; Mathew, V.; Pham, D.T.; Hwang, J.-Y.; Sun, Y.-K.; et al. Multidimensional Na4VMn0.9Cu0.1(PO4)3/C cotton-candy cathode materials for high energy Na-ion batteries. J. Mater. Chem. A 2020, 8, 12055–12068. [Google Scholar] [CrossRef]
- Wang, L.; Huang, K.-W.; Chen, J.; Zheng, J. Ultralong cycle stability of aqueous zinc-ion batteries with zinc vanadium oxide cathodes. Sci. Adv. 2019, 5, eaax4279. [Google Scholar] [CrossRef] [PubMed]
- Kundu, D.; Hosseini Vajargah, S.; Wan, L.; Adams, B.; Prendergast, D.; Nazar, L.F. Aqueous vs. nonaqueous Zn-ion batteries: Consequences of the desolvation penalty at the interface. Energy Environ. Sci. 2018, 11, 881–892. [Google Scholar] [CrossRef]
- Oberholzer, P.; Tervoort, E.; Bouzid, A.; Pasquarello, A.; Kundu, D. Oxide versus Nonoxide Cathode Materials for Aqueous Zn Batteries: An Insight into the Charge Storage Mechanism and Consequences Thereof. ACS Appl. Mater. Interfaces 2019, 11, 674–682. [Google Scholar] [CrossRef]
- Wang, H.; Jing, R.; Shi, J.; Zhang, M.; Jin, S.; Xiong, Z.; Guo, L.; Wang, Q. Mo-doped NH4V4O10 with enhanced electrochemical performance in aqueous Zn-ion batteries. J. Alloys Compd. 2021, 858, 158380. [Google Scholar] [CrossRef]
- Zhu, T.; Mai, B.; Hu, P.; Liu, Z.; Cai, C.; Wang, X.; Zhou, L. Ammonium Ion and Structural Water Co-Assisted Zn2+ Intercalation/De-Intercalation in NH4V4O10∙0.28H2O. Chin. J. Chem. 2021. [Google Scholar] [CrossRef]
- He, T.; Ye, Y.; Li, H.; Weng, S.; Zhang, Q.; Li, M.; Liu, T.; Cheng, J.; Wang, X.; Lu, J.; et al. Oxygen-deficient ammonium vanadate for flexible aqueous zinc batteries with high energy density and rate capability at −30 °C. Mater. Today 2021, 43, 53–61. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Zheng, J.; Jiang, H.; Hu, T.; Meng, C. Ammonium ion intercalated hydrated vanadium pentoxide for advanced aqueous rechargeable Zn-ion batteries. Mater. Today Energy 2020, 18, 100509. [Google Scholar] [CrossRef]







| Ammonium Vanadate Based Cathodes | Synthesis Method | Synthesis Time (h) | Maximum Discharge Capacity (mAh g−1)/(mA g−1) | Rate Capability (mAh g−1)/Rate (mA g−1) |
|---|---|---|---|---|
| NH4V4O10 [32] | Hydrothermal | 48 | ~400/300 | ~180/10,000 |
| Mo-doped NH4V4O10 [49] | Hydrothermal | 10 | ~330/100 | ~150/2000 |
| NH4V4O10.0.28H2O [50] | Hydrothermal | ~ | ~400/200 | ~112/10,000 |
| NH4V4O10-x.xH2O [51] | Hydrothermal | ~ | ~410/50 | ~120/3000 |
| (NH4)xV2O5.nH2O [52] | Hydrothermal | 48 | ~370/100 | N/A |
| NH4V4O10 [This work] | Microwave | 0.5 | ~450/100 | ~170/6400 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Soundharrajan, V.; Kim, S.; Sambandam, B.; Mathew, V.; Hwang, J.-Y.; Kim, J. Microwave-Assisted Rapid Synthesis of NH4V4O10 Layered Oxide: A High Energy Cathode for Aqueous Rechargeable Zinc Ion Batteries. Nanomaterials 2021, 11, 1905. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11081905
Kim S, Soundharrajan V, Kim S, Sambandam B, Mathew V, Hwang J-Y, Kim J. Microwave-Assisted Rapid Synthesis of NH4V4O10 Layered Oxide: A High Energy Cathode for Aqueous Rechargeable Zinc Ion Batteries. Nanomaterials. 2021; 11(8):1905. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11081905
Chicago/Turabian StyleKim, Seokhun, Vaiyapuri Soundharrajan, Sungjin Kim, Balaji Sambandam, Vinod Mathew, Jang-Yeon Hwang, and Jaekook Kim. 2021. "Microwave-Assisted Rapid Synthesis of NH4V4O10 Layered Oxide: A High Energy Cathode for Aqueous Rechargeable Zinc Ion Batteries" Nanomaterials 11, no. 8: 1905. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11081905