Encapsulation of Polyphenols from Lycium barbarum Leaves into Liposomes as a Strategy to Improve Their Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
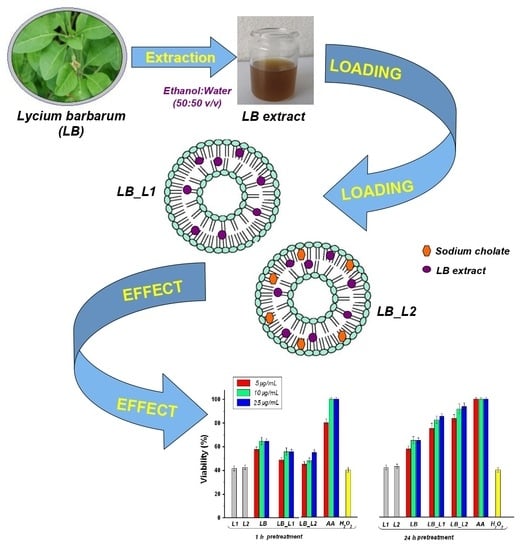
2.2. Plant Material and L. barbarum Extract Preparation
2.3. Characterization of L. barbarum Extract
2.4. Preparation of Liposomes Loaded with L. barbarum Extract
2.5. Characterization of Liposomes
2.6. In Vitro Polyphenols Release Study from Liposome Formulations
2.7. Free Radical Scavenging Activity
2.8. In Vitro Cytotoxicity Assay
2.9. Oxidative Stress Induction Assay
2.10. Uptake of Liposome by Fibroblasts
2.11. Statistical Analysis
3. Results
3.1. Characterization of L. barbarum Extract
3.2. Characterization of Liposomes Loaded with L. barbarum
3.3. In Vitro Polyphenols Release Study
3.4. Determination of H2O2 Concentration Capable of Reducing Cell Viability
3.5. Determination of the Effect of Pretreatment with Test Compounds on H2O2-Induced Cell Death
3.6. Uptake of Liposomes Loaded with L. barbarum by Fibroblasts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in effi cacy and safety of Lycium barbarum fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Ren, L.; Li, J.; Xiao, Y.; Zhang, Y.; Fan, J.; Zhang, B.; Wang, L.; Shen, X. Polysaccharide from Lycium barbarum L. leaves enhances absorption of endogenous calcium, and elevates cecal calcium transport protein levels and serum cytokine levels in rats. J. Funct. Foods 2017, 33, 227–234. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, Y.; Wang, Y.; Gao, F.; Chen, Z. Lycium barbarum: A Traditional Chinese Herb and A Promising Anti-Aging Agent. Aging Dis. 2017, 8, 778–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Xiao, J.; Fan, H.X.; Yu, Y.; He, R.-R.; Feng, X.-L.; Kurihara, H.; So, K.-F.; Yao, X.-S.; Gao, H. Polyphenols from wolfberry and their bioactivities. Food Chem. 2016, 214, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Phytosomes: Emerging Strategy in Delivery of Herbal Drugs and Nutraceuticals. Pharma Times 2009, 41, 9–12. [Google Scholar]
- Saraf, A. Applications of novel drug delivery system for herbal formulations. Fitoterapia 2010, 81, 680–689. [Google Scholar] [CrossRef]
- Yadav, M.; Bhatia, V.; Doshi, G.; Shastri, K. Novel Techniques in Herbal Drug Delivery Systems. Int. J. Pharm. Sci. Rev. Res. 2014, 28, 83–89. [Google Scholar]
- Eloy, J.O.; de Souza, M.C.; Petrilli, R.; Barcellos, J.P.A.; Lee, R.; Marchetti, J.M. Liposomes as carriers of hydrophilic small molecule drugs: Strategies to enhance encapsulation and delivery. Colloids Surf. B Biointerfaces 2014, 123, 345–363. [Google Scholar] [CrossRef]
- Chauhan, N.S.; Gowtham, R.; Gopalkrishna, B. Phytosome: Apotential phyto-phospholipid carriers for herbal drug delivery. J. Pharm. Res. 2009, 2, 1267–1270. [Google Scholar]
- Aroonsri, P.; Jintanaporn, W.; Saengrawee, S.; Wathita, P.; Supaporn, M. Anxiety and cognitive effects of quercetin liposomes in rats. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 70–78. [Google Scholar]
- El-Samaligy, M.S.; Afifi, N.N.; Mahmoud, E.A. Evaluation of hybrid liposomes-encapsulated silymarin regarding physical stability and in vivo performance. Int. J. Pharm. 2006, 319, 121–129. [Google Scholar] [CrossRef]
- Sinjari, B.; Pizzicannella, J.; D’Aurora, M.; Zappacosta, R.; Gatta, V.; Fontana, A.; Trubiani, O.; Diomede, F. Curcumin/Liposome Nanotechnology as Delivery Platform for Anti-inflammatory Activities via NFkB/ERK/pERK Pathway in Human Dental Pulp Treated With 2-HydroxyEthyl MethAcrylate (HEMA). Front. Physiol. 2019, 10, 633. [Google Scholar] [CrossRef]
- Joraholmen, M.W.; Skalko-Basnet, N.; Acharya, G.; Basnet, P. Resveratrol-loaded liposomes for topical treatment of the vaginal inflammation and infections. Eur. J. Pharm. Sci. 2015, 79, 112–121. [Google Scholar] [CrossRef] [Green Version]
- Bonechi, C.; Donati, A.; Tamasi, G.; Leone, G.; Consumi, M.; Rossi, C.; Lamponi, S.; Magnani, A. Protective effect of quercetin and rutin encapsulated liposomes on induced oxidative stress. Biophys. Chem. 2018, 233, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.B.; Singh, M.; Betageri, G.V. Encapsulation, stability and in-vitro release characteristics of liposomal formulations of colchicine. J. Pharm. Pharmacol. 1997, 49, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Lee, W.R.; Shen, S.C.; Huang, Y.L. Effect of liposome encapsulation of tea catechins on their accumulation in basal cell carcinomas. J. Dermatol. Sci. 2006, 42, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Deng, Y.; Wang, X.; Yang, B. Multivesicular liposome formulation for the sustained delivery of breviscapine. Int. J. Pharm. 2005, 301, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Sinico, C.; De Logu, A.; Lai, F.; Valenti, D.; Manconi, M.; Loy, G.; Bonsignore, L.; Fadda, A.M. Liposomal incorporation of Artemisia arborescens L. essential oil and in vitro antiviral activity. Eur. J. Pharm. Biopharm. 2005, 59, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Horvath, B.; Safranko, S.; Jokic, S.; Szechenyi, A.; Koszegi, T. Antimicrobial Activity of Chamomile Essential Oil: Effect of Different Formulations. Molecules 2019, 24, 4321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soon, S.K.; Sun, Y.K.; Bong, J.K.; Kyeong, J.K.; Geun, Y.N.; Na, R.I.; Ji, W.L.; Ji, H.H.; Junoh, K.; Soo, N.P. Cell penetrating peptide conjugated liposomes as transdermal delivery system of Polygonum aviculare L. extract. Int. J. Pharm. 2015, 483, 26–37. [Google Scholar] [CrossRef]
- Castangia, I.; Caddeo, C.; Manca, M.L.; Casu, L.; Latorre, A.C.; Díez-Sales, O.; Ruiz-Saurí, A.; Bacchetta, G.; Fadda, A.M.; Manconi, M. Delivery of liquorice extract by liposomes and hyalurosomes to protect the skin against oxidative stress injuries. Carbohydr. Polym. 2015, 134, 657–663. [Google Scholar] [CrossRef]
- Javad Asili, F.; Mosallaei, N.; Shaterzadeh, A.; Malaekeh-Nikouei, B. Preparation and characterization of liposomes containing methanol extract of aerial parts of Platycladus orientalis (L.). Avicenna J. Phytomed. 2012, 2, 17–23. [Google Scholar]
- Gibis, M.; Zeeb, B.; Weiss, J. Formation, characterization, and stability of encapsulated hibiscus extract in multilayered liposomes. Food Hydrocoll. 2014, 38, 28–39. [Google Scholar] [CrossRef]
- Bo, R.; Ma, X.; Feng, Y.; Zhu, Q.; Huang, Y.; Liu, Z.; Liu, C.; Gao, Z.; Hu, Y.; Wang, D. Optimization on conditions of Lycium barbarum polysaccharides liposome by RSM and its effects on the peritoneal macrophages function. Carbohydr. Polym. 2015, 117, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Zu, M.; Song, H.; Zhang, J.; Chen, Q.; Deng, S.; Canup, B.; Yuan, Y.; Xiao, B. Lycium barbarum lipid-based edible nanoparticles protect against experimental colitis. Colloids Surf. B Biointerfaces 2020, 187, 110747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jia, G.; Wanbin, Z.; Minghao, J.; Wei, Y.; Hao, J.; Liu, X.; Gan, Z.; Sun, A. Nanoencapsulation of zeaxanthin, extracted from Lycium barbarum L. by complex coacervation with gelatin and CMC. Food Hydrocoll. 2021, 112, 106280. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, Y.; Guo, H.; Fu, H. Evaluation of the bioaccessibility of carotenoid esters from Lycium barbarum L. in nano-emulsions: A kinetic approach. Food Res Int. 2020, 136, 109611. [Google Scholar] [CrossRef] [PubMed]
- Romanian Pharmacopoeia, X ed.; Editura Medicala: Bucuresti, Romania, 1993; Volume 1 334–335, pp. 1063–1064.
- Păvăloiu, R.-D.; Sháat, F.; Bubueanu, C.; Deaconu, M.; Neagu, G.; Sháat, M.; Anastasescu, M.; Mihailescu, M.; Matei, C.; Nechifor, G.; et al. Polyphenolic Extract from Sambucus ebulus L. Leaves Free and Loaded into Lipid Vesicles. Nanomaterials 2020, 10, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miere, F.; Vicas, S.I.; Timar, A.V.; Ganea, M.; Zdrinca, M.; Cavalu, S.; Fritea, L.; Vicas, L.; Muresan, M.; Pallag, A.; et al. Preparation and Characterization of Two Different Liposomal Formulations with Bioactive Natural Extract for Multiple Applications. Processes 2021, 9, 432. [Google Scholar] [CrossRef]
- Miere, F.; Teusdea, A.C.; Laslo, V.; Fritea, L.; Moldovan, L.; Costea, T.; Uivarosan, D.; Vicas, S.I.; Pallag, A. Natural Polymeric Beads for Encapsulation of Stellaria media Extract with Antioxidant Properties. Mater. Plast. 2019, 56, 671–679. [Google Scholar] [CrossRef]
- Sanchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Agric. Food Chem. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Mocan, A.; Zengin, G.; Simirgiotis, M.; Schafberg, M.; Mollica, A.; Vodnar, D.; Crişan, G.; Rohn, S. Functional constituents of wild and cultivated Goji (L. barbarum L.) leaves: Phytochemical characterization, biological profile, and computational studies. J. Enzyme Inhib. Med. Chem. 2017, 32, 153–168. [Google Scholar] [CrossRef] [Green Version]
- Mocan, A.; Vlase, L.; Vodnar, D.C.; Bischin, C.; Hanganu, D.; Gheldiu, A.M.; Oprean, R.; Silaghi-Dumitrescu, R.; Crisan, G. Polyphenolic Content, Antioxidant and Antimicrobial Activities of Lycium barbarum L. and Lycium chinense Mill. Leaves. Molecules 2014, 19, 10056–10073. [Google Scholar] [CrossRef] [PubMed]
- Bajalan, I.; Mohammadi, M.; Alaei, M.; Pirbalouti, A.G. Total phenolic and flavonoid contents and antioxidant activity of extracts from different populations of lavandin. Ind. Crops Prod. 2016, 87, 255–260. [Google Scholar] [CrossRef]
- Bilgin, M.S.; Ahin, S. Effects of geographical origin and extraction methods on total phenolic yield of olive tree (Olea europaea) leaves. J. Taiwan Inst. Chem. Eng. 2013, 44, 8–12. [Google Scholar] [CrossRef]
- Malek, S.N.; Phang, C.W.; Ibrahim, H.; Abdul Wahab, N.; Sim, K.S. Phytochemical and cytotoxic investigations of Alpinia mutica rhizomes. Molecules 2011, 16, 583–589. [Google Scholar] [CrossRef] [Green Version]
- Malek, S.N.; Lee, G.S.; Hong, S.L.; Yaacob, H.; Wahab, N.A.; Weber, F.; Shah, S.A. Phytochemical and Cytotoxic Investigations of Curcuma mangga Rhizomes. Molecules 2011, 16, 4539–4548. [Google Scholar] [CrossRef] [Green Version]
- Gibis, M.; Vogt, E.; Weiss, J. Encapsulation of polyphenolic grape seed extract in polymer-coated liposomes. Food Funct. 2012, 3, 246–254. [Google Scholar] [CrossRef]
- Gupta, A.; Aggarwal, G.; Singla, S.; Arora, R. Transfersomes: A Novel Vesicular Carrier for Enhanced Transdermal Delivery of Sertraline: Development, Characterization, and Performance Evaluation. Sci. Pharm. 2012, 80, 1061–1080. [Google Scholar] [CrossRef] [Green Version]
- Gharib, R.; Amal Najjar, A.; Auezova, L.; Catherine Charcosset, C.; Greige-Gerges, H. Interaction of selected phenylpropenes with dipalmitoylphosphatidylcholine membrane and their relevance to antibacterial activity. J. Membrane Biol. 2017, 250, 259–271. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Poloniae Pharm. Drug Res. 2010, 67, 217–223. [Google Scholar]
- Gibis, M.; Ruedt, C.; Weiss, J. In vitro release of grape-seed polyphenols encapsulated from uncoated and chitosan-coated liposomes. Food Res. Int. 2016, 88, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.M.; Valento, P.; Pereira, J.A.; Andrad, P.B. Phenolics: From chemistry to biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Vinson, J.A.; Su, X.; Zubik, L.; Bose, P. Phenol antioxidant quantity and quality in foods: Fruits. J. Agric. Food Chem. 2001, 49, 5315–5321. [Google Scholar] [CrossRef]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef] [Green Version]
- Hancock, J.T.; Desikan, R.; Neill, S.J. Role of Reactive Oxygen Species in Cell Signaling Pathways. Biochem. Biomed. Asp. Oxidative Modif. 2001, 29, 345–350. [Google Scholar]
- Jones, D.P. Redox Potential of GSH/GSSG Couple: Assay and Biological Significance. Methods Enzymol. 2000, 348, 93–112. [Google Scholar]
- McCord, J.M.; Fridovich, I. The Reduction of Cytochrome C by Milk Xanthinse Oxidase. J. Biol. Chem. 1968, 243, 5733–5760. [Google Scholar] [CrossRef]
- Galano, A.; Mazzone, G.; Alvarez-Diduk, R.; Marino, T.; Alvarez-Idaboy, J.R.; Russo, N. The food antioxidants: Chemical insights at the molecular level. Annu. Rev. Food Sci. Technol. 2016, 7, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Alvarez-Idaboy, J.R. A computational methodology for accurate predictions of rate constants in solution: Application to the assessment of primary antioxidant activity. J. Comp. Chem. 2013, 34, 2430–2445. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Turkez, H.; Sozio, P.; Geyikoglu, F.; Tatar, A.; Hacimuftuoglu, A.; Di Stefano, A. Cell Neuroprotective effects of farnesene against hydrogen peroxide-induced neurotoxicity in vitro. Mol. Neurobiol. 2014, 34, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Simões, S.; Slepushkin, V.; Düzgünes, N.; de Lima, M.C.P. On the mechanisms of internalization and intracellular delivery mediated by pH-sensitive liposomes. Biochim. Biophys. Acta (BBA) Biomembr. 2001, 1515, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Alshehri, A.; Grabowska, A.; Snow, S. Pathways of cellular internalisation of liposomes delivered siRNA and effects on siRNA engagement with target mRNA and silencing in cancer cells. Sci. Rep. 2018, 8, 3748. [Google Scholar] [CrossRef]




| Sample Code | Particle Size (nm) | PDI | EE (%) | EE—1 Month (%) | EE—2 Months (%) | EE—3 Months (%) |
|---|---|---|---|---|---|---|
| LB_L1 | 141.6 ± 2.360 | 0.187 ± 0.001 | 84.60 ± 2.230 | 83.75 ± 1.030 | 81.45 ± 2.150 | 79.85 ± 1.030 |
| LB_L2 | 195.9 ± 1.220 | 0.114 ± 0.001 | 75.25 ± 1.761 | 74.53 ± 1.241 | 72.93 ± 1.171 | 71.01 ± 1.465 |
| L1 | 85.4 ± 0.341 | 0.300 ± 0.003 | - | - | - | - |
| L2 | 105.2 ± 2.042 | 0.279 ± 0.005 | - | - | - | - |
| Sample Code | Weibull | Korsmeyer–Peppas | ||||
|---|---|---|---|---|---|---|
| a | b | R2 | n | k | R2 | |
| L. barbarum | 0.937 | 0.402 | 0.967 | - | - | - |
| L1_LB | 0.541 | 0.541 | 0.975 | 0.215 | 1.609 | 0.974 |
| L2_LB | 0.158 | 0.152 | 0.951 | 0.180 | 1.186 | 0.985 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Păvăloiu, R.-D.; Sha’at, F.; Neagu, G.; Deaconu, M.; Bubueanu, C.; Albulescu, A.; Sha’at, M.; Hlevca, C. Encapsulation of Polyphenols from Lycium barbarum Leaves into Liposomes as a Strategy to Improve Their Delivery. Nanomaterials 2021, 11, 1938. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11081938
Păvăloiu R-D, Sha’at F, Neagu G, Deaconu M, Bubueanu C, Albulescu A, Sha’at M, Hlevca C. Encapsulation of Polyphenols from Lycium barbarum Leaves into Liposomes as a Strategy to Improve Their Delivery. Nanomaterials. 2021; 11(8):1938. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11081938
Chicago/Turabian StylePăvăloiu, Ramona-Daniela, Fawzia Sha’at, Georgeta Neagu, Mihaela Deaconu, Corina Bubueanu, Adrian Albulescu, Mousa Sha’at, and Cristina Hlevca. 2021. "Encapsulation of Polyphenols from Lycium barbarum Leaves into Liposomes as a Strategy to Improve Their Delivery" Nanomaterials 11, no. 8: 1938. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11081938