The Influence of Temperature Increase on the Toxicity of Mercury Remediated Seawater Using the Nanomaterial Graphene Oxide on the Mussel Mytilus galloprovincialis
Abstract
:1. Introduction
1.1. Impacts of Mercury in Marine Ecosystems
1.2. Strategies to Remediate Contaminated Waters
1.3. Impacts of Temperature in Marine Organisms
2. Materials and Methods
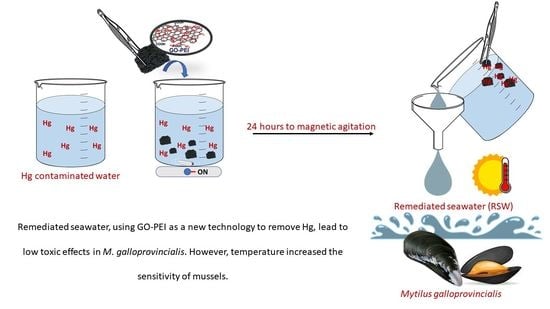
2.1. Experiment Setup
2.2. Graphene Oxide with Ethyleneimine Polymer
2.3. Mercury Quantification
2.4. Biological Responses: Metabolic Capacity, Oxidative Stress and Neurotoxicity Biomarkers
2.5. Biological Responses: Histopathological Measurements
2.6. Integrated Biomarker Response
2.7. Statistical Analysis
2.8. Multivariate Analysis
3. Results
3.1. Mercury Quantification
3.2. Biological Assays: Metabolic Capacity, Oxidative Stress and Neurotoxicity Biomarkers
3.3. Biological Responses: Histopathological Measurements
3.4. Integrated Biomarker Response
3.5. Multivariate Analysis
4. Discussion
4.1. Impacts of Temperature in Mussels Exposed to Control Treatment
4.2. Impacts of Temperature in Mussels Exposed to Hg Treatments
4.3. Impacts of Temperature in Mussels Exposed to GO–PEI Treatment
4.4. Impacts of Temperature in Mussels Exposed to Remediated Seawater
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coppola, F.; Bessa, A.; Henriques, B.; Russo, T.; Soares, A.M.V.M.; Figueira, E.; Marques, P.; Polese, G.; Di Cosmo, A.; Pereira, E.; et al. Oxidative stress, metabolic and histopathological alterations in mussels exposed to remediated seawater by GO‒PEI after contamination with mercury. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 243, 110674. [Google Scholar] [CrossRef]
- ATSDR. Agency for Toxic Substances and Disease Registry Priority List of Hazardous Substances, Priority List of Hazardous Substances, Agency for Toxic Substances and Disease Registry; US Public Health Service: Atalanta, GA, USA, 2019.
- Guilherme, S.; Válega, M.; Pereira, M.E.; Santos, M.A.; Pacheco, M. Antioxidant and biotransformation responses in Liza aurata under environmental mercury exposure—Relationship with mercury accumulation and implications for public health. Mar. Pollut. Bull. 2008, 56, 845–859. [Google Scholar] [CrossRef]
- Spada, L.; Annicchiarico, C.; Cardellicchio, N.; Giandomenico, S.; Di Leo, A. Mercury and methylmercury concentrations in Mediterranean seafood and surface sediments, intake evaluation and risk for consumers. Int. J. Hyg. Environ. Health 2012, 215, 418–426. [Google Scholar] [CrossRef]
- Vaselli, O.; Nisi, B.; Rappuoli, D.; Bianchi, F.; Cabassi, J.; Venturi, S.; Tassi, F.; Raco, B. Geochemical characterization of the ground waters from the former Hg-mining area of Abbadia San Salvatore (Mt. Amiata, central Italy): Criticalities and perspectives for the reclamation process. Ital. J. Geosci. 2015, 134, 304–322. [Google Scholar] [CrossRef]
- Cooper, K.; Marshall, L.; Vanderlinden, L.; Ursitti, F. Early Exposures to Hazardous Chemicals/Pollution and Associations with Chronic Disease: A Scoping Review, a Report from the Canadian Environmental Law Association, the Ontario College of Family Physicians and the Environmental Health Institute of Canada; CELA Publication: Toronto, Canada, 2011. [Google Scholar]
- Davydova, S. Heavy metals as toxicants in big cities. Microchem. J. 2005, 79, 133–136. [Google Scholar] [CrossRef]
- Donnici, S.; Serandrei-Barbero, R.; Canali, G. Evidence of climatic changes in the Venetian Coastal Plain (Northern Italy) during the last 40,000 years. Sediment. Geol. 2012, 281, 139–150. [Google Scholar] [CrossRef]
- Pereira, M.; Abreu, S.; Coelho, J.; Pato, P.; Pardal, M.; Duarte, A. Influence of tidal resuspension on seston lithogenic and biogenic partitioning in shallow estuarine systems: Implications for sampling. Mar. Pollut. Bull. 2008, 56, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Randall, P.M.; Chattopadhyay, S. Mercury contaminated sediment sites—An evaluation of remedial options. Environ. Res. 2013, 125, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.A.D.S.; Spencer, K.; Kloas, W.; Toffolon, M.; Zarfl, C. Metal fate and effects in estuaries: A review and conceptual model for better understanding of toxicity. Sci. Total. Environ. 2016, 541, 268–281. [Google Scholar] [CrossRef]
- Portela, J.F.; de Souza, J.P.R.; de Sousa Tonhá, M.; Bernardi, J.V.E.; Garnier, J.; Souzade, J.R. Evaluation of total mercury in sediments of the Descoberto river environmental protection area—Brazil. Int. J. Environ. Res. Public Health 2020, 17, 154. [Google Scholar] [CrossRef] [Green Version]
- Sunderland, E.; Krabbenhoft, D.P.; Moreau, J.W.; Strode, S.A.; Landing, W.M. Mercury sources, distribution, and bioavailability in the North Pacific Ocean: Insights from data and models. Glob. Biogeochem. Cycles 2009, 23, GB2010. [Google Scholar] [CrossRef] [Green Version]
- Faganeli, J.; Hines, M.E.; Covelli, S.; Emili, A.; Giani, M. Mercury in lagoons: An overview of the importance of the link between geochemistry and biology. Estuar. Coast. Shelf Sci. 2012, 113, 126–132. [Google Scholar] [CrossRef]
- Gworek, B.; Bemowska-Kałabun, O.; Kijeńska, M.; Wrzosek-Jakubowska, J. Mercury in Marine and Oceanic Waters—A Review. Water Air Soil Pollut. 2016, 227, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelidis, M.O.; Catsiki, V.A. Metal Bioavailability and Bioaccumulation in the Marine Environement: Methodological Questions; CIESM Workshop Monograph: Monaco City, Monaco, 2002. [Google Scholar]
- Casas, S.; Bacher, C. Modelling trace metal (Hg and Pb) bioaccumulation in the Mediterranean mussel, Mytilus galloprovincialis, applied to environmental monitoring. J. Sea Res. 2006, 56, 168–181. [Google Scholar] [CrossRef] [Green Version]
- Galvão, P.M.A.; Rebelo, M.F.; Guimarães, J.R.D.; Torres, J.P.M.; Malm, O. Bioacumulação de metais em moluscos bivalves: Aspectos evolutivos e ecológicos a serem considerados para a biomonitorização de ambientes marinhos. Braz. J. Aquat. Sci. Technol. 2009, 13, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.; Piló, D.; Carvalho, S.; Araújo, O.; Guilherme, S.; Santos, M.A.; Vale, C.; Pereia, F.; Pacheco, M.; Pereira, P. Metal bioaccumulation and oxidative stress profiles in Ruditapes philippinarum e insights towards its suitability as bioindicator of estuarine metal contamination. Ecol. Indicat. 2018, 95, 1087–1099. [Google Scholar] [CrossRef]
- Morrison, R.J.; Brown, L.P. Trace metals in Fangauta Lagoon, Kingdom of Tonga. Mar. Pollut. Bull. 2003, 46, 139–152. [Google Scholar] [CrossRef]
- Moschino, V.; Delaney, E.; Da Ros, L. Assessing the significance of Ruditapes philippinarum as a sentinel for sediment pollution: Bioaccumulation and biomarker responses. Environ. Pollut. 2012, 171, 52–60. [Google Scholar] [CrossRef]
- Hamza-Chaffai, A. Usefulness of Bioindicators and Biomarkers in Pollution Biomonitoring. Int. J. Biotechnol. Wellness Ind. 2014, 3, 19–26. [Google Scholar] [CrossRef]
- Fernández, B.; Campillo, J.; Martínez-Gómez, C.; Benedicto, J. Antioxidant responses in gills of mussel (Mytilus galloprovincialis) as biomarkers of environmental stress along the Spanish Mediterranean coast. Aquat. Toxicol. 2010, 99, 186–197. [Google Scholar] [CrossRef]
- Cuevas, N.; Zorita, I.; Costa, P.M.; Franco, J.; Larreta, J. Development of histopathological indices in the digestive gland and gonad of mussels: Integration with contamination levels and effects of confounding factors. Aquat. Toxicol. 2015, 162, 152–164. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, R.; Li, C.; Bao, Y. Mercury exposure modulates antioxidant enzymes in gill tissue and hemocytes of Venerupis philippinarum. Invertebr. Surviv. J. 2014, 11, 298–308. [Google Scholar]
- Coppola, F.; Henriques, B.; Soares, A.M.V.M.; Figueira, E.; Pereira, E.; Freitas, R. Influence of temperature rise on the recovery capacity of Mytilus galloprovincialis exposed to mercury pollution. Ecol. Indic. 2018, 93, 1060–1069. [Google Scholar] [CrossRef]
- Freitas, R.; Coppola, F.; Henriques, B.; Wrona, F.; Figueira, E.; Pereira, E.; Soares, A.M.V.M. Does pre-exposure to warming conditions increase Mytilus galloprovincialis tolerance to Hg contamination? Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 203, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Journals, I.; Sonawane, S.M. Effect of Heavy Metals on Gills of Fresh Water Bivalve Lamellidens marginalis. IOSR J. Environ. Sci. Toxicol. Food Technol. 2015, 9, 5–11. [Google Scholar] [CrossRef]
- Ali, I.; Khan, T.A.; Asim, M. Removal of arsenate from groundwater by electrocoagulation method. Environ. Sci. Pollut. Res. 2011, 19, 1668–1676. [Google Scholar] [CrossRef]
- Anjum, M.; Miandad, R.; Waqas, M.; Gehany, F.; Barakat, M. Remediation of wastewater using various nano-materials. Arab. J. Chem. 2019, 12, 4897–4919. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Lin, D.; Ning, K.; Sui, Y.; Hu, M.; Lu, W.; Wang, Y. Hemocyte responses of the thick shell mussel Mytilus coruscus exposed to nano-TiO2 and seawater acidification. Aquat. Toxicol. 2016, 180, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Feng, C.; Zhang, Z.; Yang, S.; Sugiura, N. Treatment of nitrate contaminated water using an electrochemical method. Bioresour. Technol. 2010, 101, 6553–6557. [Google Scholar] [CrossRef] [PubMed]
- Coppola, F.; Bessa, A.; Henriques, B.; Russo, T.; Soares, A.M.V.M.; Figueira, E.; Pereira, E.; Marques, P.; Polese, G.; Freitas, R. The Role of Temperature on the Impact of Remediated Water towards Marine Organisms. Water 2020, 12, 2148. [Google Scholar] [CrossRef]
- Coppola, F.; Tavares, D.S.; Henriques, B.; Monteiro, R.; Trindade, T.; Figueira, E.; Soares, A.M.V.M.; Pereira, E.; Freitas, R. Can water remediated by manganese spinel ferrite nanoparticles be safe for marine bivalves? Sci. Total Environ. 2020, 723, 137798. [Google Scholar] [CrossRef] [PubMed]
- Coppola, F.; Tavares, D.; Henriques, B.; Monteiro, R.; Trindade, T.; Soares, A.M.; Figueira, E.; Polese, G.; Pereira, E.; Freitas, R. Remediation of arsenic from contaminated seawater using manganese spinel ferrite nanoparticles: Ecotoxicological evaluation in Mytilus galloprovincialis. Environ. Res. 2019, 175, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Da̧browski, A.; Hubicki, Z.; Podkoscielny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef]
- Gehrke, I.; Geiser, A.; Somborn-Schulz, A. Innovations in nanotechnology for water treatment. Nanotechnol. Sci. Appl. 2015, 8, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, D.; Pittman, C.U. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Chen, M.-Y.; Yang, H.-S.; Delaporte, M.; Zhao, S.-J.; Xing, K. Immune responses of the scallop Chlamys farreri after air exposure to different temperatures. J. Exp. Mar. Biol. Ecol. 2007, 345, 52–60. [Google Scholar] [CrossRef]
- Jackson, B.P.; Bugge, D.; Ranville, J.; Chen, C.Y. Bioavailability, Toxicity, and Bioaccumulation of Quantum Dot Nanoparticles to the Amphipod Leptocheirus plumulosus. Environ. Sci. Technol. 2012, 46, 5550–5556. [Google Scholar] [CrossRef] [Green Version]
- Paul, B.; Parashar, V.; Mishra, A. Graphene in the Fe3O4 nano-composite switching the negative influence of humic acid coating into an enhancing effect in the removal of arsenic from water. Environ. Sci. Water Res. Technol. 2014, 1, 77–83. [Google Scholar] [CrossRef]
- Vilela, D.; Parmar, J.; Zeng, Y.; Zhao, Y.; Sánchez, S. Graphene-Based Microbots for Toxic Heavy Metal Removal and Recovery from Water. Nano Lett. 2016, 16, 2860–2866. [Google Scholar] [CrossRef] [Green Version]
- Henriques, B.; Gonçalves, G.; Emami, N.; Pereira, E.; Vila, M.; Marques, P. Optimized graphene oxide foam with enhanced performance and high selectivity for mercury removal from water. J. Hazard. Mater. 2016, 301, 453–461. [Google Scholar] [CrossRef]
- Yang, K.; Wang, J.; Chen, X.; Zhao, Q.; Ghaffar, A.; Chen, B. Application of gra- phene-based materials in water purification: From the nanoscale to specific devices. Environ. Sci. Nano. 2018, 5, 1264–1297. [Google Scholar] [CrossRef]
- Ali, I.; Alharbi, O.M.L.; Tkachev, A.; Galunin, E.; Burakov, A.; Grachev, V. Water treatment by new-generation graphene materials: Hope for bright future. Environ. Sci. Pollut. Res. 2018, 25, 7315–7329. [Google Scholar] [CrossRef]
- Nupearachchi, C.N.; Mahatantila, K.; Vithanage, M. Application of graphene for decontamination of water; Implications for sorptive removal. Groundw. Sustain. Dev. 2017, 5, 206–215. [Google Scholar] [CrossRef]
- Zhang, Z.-W.; Xu, X.-R.; Sun, Y.; Yu, S.; Chen, Y.-S.; Peng, J.-X. Heavy metal and organic contaminants in mangrove ecosystems of China: A review. Environ. Sci. Pollut. Res. 2014, 21, 11938–11950. [Google Scholar] [CrossRef]
- Bessa, A.; Henriques, B.; Gonçalves, G.; Irurueta, G.; Pereira, E.; Marques, P.A.A.P. Graphene oxide / polyethyleneimine aerogel for high-performance mercury sorption from natural waters. Chem. Eng. J. 2020, 398, 125587. [Google Scholar] [CrossRef]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological interactions of graphene family nanomaterials—An interdisciplinary review. Chem. Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef] [Green Version]
- Petersen, E.J.; Pinto, R.A.; Mai, D.; Landrum, P.F.; Weber, J.W.J. Influence of Polyethyleneimine Graftings of Multi-Walled Carbon Nanotubes on their Accumulation and Elimination by and Toxicity to Daphnia magna. Environ. Sci. Technol. 2011, 45, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, M.; Incze, L.; Hayhoe, K.; Mountain, D.; Manning, J. Potential climate change impacts on Atlantic cod (Gadus morhua) off the northeastern USA. Mitig. Adapt. Strat. Glob. Chang. 2007, 13, 453–466. [Google Scholar] [CrossRef]
- IPCC. Aquecimento Global de 1.5 °C. Sumário Para Formuladores de Políticas; IPCC: Geneva, Switzerland, 2018; Available online: https://www.ipcc.ch/site/assets/uploads/2019/07/SPM-Portuguese-version.pdf (accessed on 20 February 2019).
- Berthelin, C.; Kellner, K.; Mathieu, M. Storage metabolism in the Pacific oyster (Crassostrea gigas) in relation to summer mortalities and reproductive cycle (West Coast of France). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2000, 125, 359–369. [Google Scholar] [CrossRef]
- Hiebenthal, C.; Philipp, E.; Eisenhauer, A.; Wahl, M. Interactive effects of temperature and salinity on shell formation and general condition in Baltic Sea Mytilus edulis and Arctica islandica. Aquat. Biol. 2012, 14, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Mackenzie, C.; Lynch, S.A.; Culloty, S.C.; Malham, S.K. Future Oceanic Warming and Acidification Alter Immune Response and Disease Status in a Commercial Shellfish Species, Mytilus edulis L. PLoS ONE 2014, 9, e99712. [Google Scholar] [CrossRef] [Green Version]
- Mubiana, V.K.; Blust, R. Effects of temperature on scope for growth and accumulation of Cd, Co, Cu and Pb by the marine bivalve Mytilus edulis. Mar. Environ. Res. 2007, 63, 219–235. [Google Scholar] [CrossRef] [Green Version]
- Sokolova, I.M.; Frederich, M.; Bagwe, R.; Lannig, G.; Sukhotin, A. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 2012, 79, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.; Coppola, F.; De Marchi, L.; Codella, V.; Pretti, C.; Chiellini, F.; Morelli, A.; Polese, G.; Soares, A.M.; Figueira, E. The influence of Arsenic on the toxicity of carbon nanoparticles in bivalves. J. Hazard. Mater. 2018, 358, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Freitas, R.; Figueira, E.; Ghirardini, A.V.; Soares, A.M.V.M.; Radaelli, M.; Guida, M.; Libralato, G. Combined effects of arsenic, salinity and temperature on Crassostrea gigas embryotoxicity. Ecotoxicol. Environ. Saf. 2018, 147, 251–259. [Google Scholar] [CrossRef]
- Maulvault, A.L.; Barbosa, V.L.; Alves, R.; Anacleto, P.; Camacho, C.; Cunha, S.; Fernandes, J.; Ferreira, P.P.; Rosa, R.; Marques, A.; et al. Integrated multi-biomarker responses of juvenile seabass to diclofenac, warming and acidification co-exposure. Aquat. Toxicol. 2018, 202, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Matozzo, V.; Chinellato, A.; Munari, M.; Bressan, M.; Marin, M.G. Can the combination of decreased pH and increased temperature values induce oxidative stress in the clam Chamelea gallina and the mussel Mytilus galloprovincialis? Mar. Pollut. Bull. 2013, 72, 34–40. [Google Scholar] [CrossRef]
- Kefaloyianni, E.; Gourgou, E.; Ferle, V.; Kotsakis, E.; Gaitanaki, C.; Beis, I. Acute thermal stress and various heavy metals induce tissue-specific pro-or anti-apoptotic events via the p38-MAPK signal transduction pathway in Mytilus galloprovincialis (Lam.). J. Exp. Biol. 2005, 208, 4427–4436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verlecar, X.N.; Jena, K.B.; Chainy, G. Biochemical markers of oxidative stress in Perna viridis exposed to mercury and temperature. Chem. Interact. 2007, 167, 219–226. [Google Scholar] [CrossRef]
- Velez, C.; Figueira, E.; Soares, A.M.; Freitas, R. Effects of seawater temperature increase on economically relevant native and introduced clam species. Mar. Environ. Res. 2017, 123, 62–70. [Google Scholar] [CrossRef]
- Attig, H.; Kamel, N.; Sforzini, S.; Dagnino, A.; Jamel, J.; Boussetta, H.; Viarengo, A.; Banni, M. Effects of thermal stress and nickel exposure on biomarkers responses in Mytilus galloprovincialis (Lam). Mar. Environ. Res. 2014, 94, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Ivanina, A.V.; Taylor, C.; Sokolova, I.M. Effects of elevated temperature and cadmium exposure on stress protein response in eastern oysters Crassostrea virginica (Gmelin). Aquat. Toxicol. 2009, 91, 245–254. [Google Scholar] [CrossRef]
- Freitas, R.; Leite, C.; Pinto, J.; Costa, M.; Monteiro, R.; Henriques, B.; Di Martino, F.; Coppola, F.; Soares, A.M.; Solé, M.; et al. The influence of temperature and salinity on the impacts of lead in Mytilus galloprovincialis. Chemosphere 2019, 235, 403–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lannig, G.; Flores, J.F.; Sokolova, I. Temperature-dependent stress response in oysters, Crassostrea virginica: Pollution reduces temperature tolerance in oysters. Aquat. Toxicol. 2006, 79, 278–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokolova, I.; Lannig, G. Interactive effects of metal pollution and temperature on metabolism in aquatic ectotherms: Implications of global climate change. Clim. Res. 2008, 37, 181–201. [Google Scholar] [CrossRef] [Green Version]
- Pirone, G.; Coppola, F.; Pretti, C.; Soares, A.; Solé, M.; Freitas, R. The effect of temperature on Triclosan and Lead exposed mussels. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2019, 232, 42–50. [Google Scholar] [CrossRef]
- IPCC. IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse gas fluxes in Terrestrial Ecosystems; IPCC: Geneva, Switzerland, 2019; pp. 1–472. [Google Scholar]
- Official Journal of the European Union. Directive 2013/39/EU, 2013. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/ EC as Regards Priority Substances in the Field of Water Policy. 2013, pp. 1–17. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:226:0001:0017:EN:PDF (accessed on 19 February 2019).
- Celo, V.; Lean, D.R.S.; Scott, S.L. Abiotic methylation of mercury in the aquatic environment. Sci. Total. Environ. 2006, 368, 126–137. [Google Scholar] [CrossRef]
- Leite, C.; Coppola, F.; Monteiro, R.; Russo, T.; Polese, G.; Lourenço, M.; Silva, M.R.F.; Ferreira, P.; Soares, A.M.V.M.; Freitas, R.; et al. Biochemical and histopathological impacts of rutile and anatase (TiO2 forms) in Mytilus galloprovincialis. Sci. Total. Environ. 2020, 719, 134886. [Google Scholar] [CrossRef] [Green Version]
- Costley, C.T.; Mossop, K.F.; Dean, J.R.; Garden, L.M.; Marshall, J.; Carroll, J. Determination of mercury in environmental and biological samples using pyrolysis atomic absorption spectrometry with gold amalgamation. Anal. Chim. Acta 2000, 405, 179–183. [Google Scholar] [CrossRef]
- De Coen, W.M.; Janssen, C. The use of biomarkers in Daphnia magna toxicity testing. IV. Cellular Energy Allocation: A new methodology to assess the energy budget of toxicant-stressed Daphnia populations. J. Aquat. Ecosyst. Stress Recover. 1997, 6, 43–55. [Google Scholar] [CrossRef]
- King, F.D.; Packard, T.T. Respiration and the respiratory electron transport in marine zooplankton. Limnol. Oceanogr. 1975, 20, 2849–2854. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Johansson, L.H.; Borg, L.A.H. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferase AA from rat liver. Arch. Biochem. Biophys. 1976, 175, 710–716. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Mesquita, A.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.; Marcos, J.C. Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Pinto, J.; Costa, M.; Leite, C.; Borges, C.; Coppola, F.; Henriques, B.; Monteiro, R.; Russo, T.; Di Cosmo, A.; Soares, A.M.; et al. Ecotoxicological effects of lanthanum in Mytilus galloprovincialis: Biochemical and histopathological impacts. Aquat. Toxicol. 2019, 211, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.M.; Carreira, S.; Costa, M.H.; Caeiro, S. Development of histopathological indices in a commercial marine bivalve (Ruditapes decussatus) to determine environmental quality. Aquat. Toxicol. 2013, 126, 442–454. [Google Scholar] [CrossRef]
- Beliaeff, B.; Burgeot, T. Integrated biomarker response: A useful tool for ecological risk assessment. Environ. Toxicol. Chem. 2002, 21, 1316–1322. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA for PRIMER: Guide to Software and Statistical Methods; University of Auckland and PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Anacleto, P.; Maulvault, A.L.; Bandarra, N.; Repolho, T.; Nunes, M.L.; Rosa, R.; Marques, A. Effect of warming on protein, glycogen and fatty acid content of native and invasive clams. Food Res. Int. 2014, 64, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Coppola, F.; Almeida Henriques, B.; Soares, A.; Figueira, E.; Pereira, E.; Freitas, R. Biochemical impacts of Hg in Mytilus galloprovincialis under present and predicted warming scenarios. Sci. Total. Environ. 2017, 601–602, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Figueira, E.; Pecora, I.L.; Soares, A.; Freitas, R. Biochemical alterations in native and exotic oyster species in Brazil in response to increasing temperature. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 191, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Fearman, J.; Moltschaniwskyj, N. Warmer temperatures reduce rates of gametogenesis in temperate mussels, Mytilus galloprovincialis. Aquaculture 2010, 305, 20–25. [Google Scholar] [CrossRef]
- Nardi, A.; Mincarelli, L.F.; Benedetti, M.; Fattorini, D.; D’Errico, G.; Regoli, F. Indirect effects of climate changes on cadmium bioavailability and biological effects in the Mediterranean mussel Mytilus galloprovincialis. Chemosphere 2017, 169, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Durán, E.G.; Cuaya, M.P.; Gutiérrez, M.V.; León, J.A. Effects of Temperature and pH on the Oxidative Stress of Benthic Marine Invertebrates. Biol. Bull. 2018, 45, 610–616. [Google Scholar] [CrossRef]
- Marigómez, I.; Múgica, M.; Izagirre, U.; Sokolova, I. Chronic environmental stress enhances tolerance to seasonal gradual warming in marine mussels. PLoS ONE 2017, 12, e0174359. [Google Scholar] [CrossRef] [Green Version]
- Matoo, O.B.; Ivanina, A.V.; Ullstad, C.; Beniash, E.; Sokolova, I. Interactive effects of elevated temperature and CO2 levels on metabolism and oxidative stress in two common marine bivalves (Crassostrea virginica and Mercenaria mercenaria). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 164, 545–553. [Google Scholar] [CrossRef]
- Vratsistas, A.; Vafidis, D.; Exadactylos, A.; Feidantsis, K.; Michaelidis, B. Investigation of the oxidative stress response of marine bivalve Mytilus galloprovincialis during long-term exposure to high temperature. In Proceedings of the 2nd International Congress on Applied Ichthyology and Aquatic Environment—HydroMediT, Messolonghi, Greece, 10–12 November 2016; pp. 346–349. [Google Scholar]
- Pandey, A.; Shanthanagouda, A.H.; Pathak, D.; Singh, A. Histopathological effects in gills of freshwater mussels, Lamellidens marginalis exposed to mercury chloride. Save Nat. Surviv. 2016, 11, 2277–2280. [Google Scholar]
- Boukadida, K.; Cachot, J.; Clérandeaux, C.; Gourves, P.-Y.; Banni, M. Early and efficient induction of antioxidant defense system in Mytilus galloprovincialis embryos exposed to metals and heat stress. Ecotoxicol. Environ. Saf. 2017, 138, 105–112. [Google Scholar] [CrossRef]
- Velez, C.; Galvao, P.; Longo, R.; Malm, O.; Soares, A.; Figueira, E.; Freitas, R. Ruditapes philippinarum and Ruditapes decussatus under Hg environmental contamination. Environ. Sci. Pollut. Res. 2015, 22, 11890–11904. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Wang, W. Mercury accumulation in marine bivalves: Influences of biodynamics and feeding niche. Environ. Pollut. 2011, 159, 2500–2506. [Google Scholar] [CrossRef] [PubMed]
- Morosetti, B.; Freitas, R.; Pereira, E.; Hamza, H.; Andrade, M.; Coppola, F.; Maggioni, D.; Della Torre, C. Will temperature rise change the biochemical alterations induced in Mytilus galloprovincialis by cerium oxide nanoparticles and mercury? Environ. Res. 2020, 188, 109778. [Google Scholar] [CrossRef]
- De Marchi, L.; Neto, V.; Pretti, C.; Figueira, E.; Chiellini, F.; Morelli, A.; Soares, A.M.V.M.; Freitas, R. Toxic effects of multi-walled carbon nanotubes on bivalves: Comparison between functionalized and nonfunctionalized nanoparticles. Sci. Total. Environ. 2018, 622–623, 1532–1542. [Google Scholar] [CrossRef] [PubMed]







| Conditions | Description |
|---|---|
| CTL 17 | Hg 0.0 µg/L + GO–PEI 0.0 mg/L at 17 °C |
| CTL 21 | Hg 0.0 µg/L + GO–PEI 0.0 mg/L at 21 °C |
| GO–PEI | GO–PEI 10 mg/L + Hg 0.0 µg/L at 21 °C |
| GO–PEI + Hg | GO–PEI 10 mg/L + Hg 50 µg/L at 21 °C |
| Hg | Hg 50 µg/L + GO–PEI 0.0 mg/L at 21 °C |
| RSW | Remediated Seawater previously contaminated with Hg (50 µg/L) and decontaminated by GO‒PEI (10 mg/L) for 24 h at 21 °C |
| Conditions | Hg Water Concentration | Mussel’s Hg Concentration |
|---|---|---|
| CTL 17 | <LOQ | 0.17 ± 0.027 A |
| CTL 21 | <LOQ | 0.08 ± 0.03 B |
| GO–PEI | <LOQ | 0.09 ± 0.01 B |
| GO–PEI + Hg | 52.1 ± 2.2 A | 13.09 ± 4.502 C |
| Hg | 52.4 ± 2.8 A | 16.19 ± 1.052 C |
| RSW | 9.6 ± 1.5 B | 6.09 ± 1.73 D |
| ETS | SOD | CAT | GSTs | LPO | PC | GSH/GSSG | AChE | Gills | Digestive Tubules | |
|---|---|---|---|---|---|---|---|---|---|---|
| CTL 17 vs. CTL 21 | 0.1948 | 0.3809 | 0.3809 | 0.0003 | 0.0889 | 0.0001 | 0.0001 | 0.0001 | 0.0321 | 0.0001 |
| CTL 17 vs. GO‒PEI | 0.0654 | 0.0093 | 0.0093 | 0.0001 | 0.0026 | 0.0001 | 0.0001 | 0.0014 | 0.0001 | 0.0001 |
| CTL 17 vs. GO–PEI + Hg | 0.1849 | 0.6506 | 0.6506 | 0.0001 | 0.0482 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| CTL 17 vs. Hg | 0.7773 | 0.0379 | 0.0379 | 0.0032 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| CTL 17 vs. RSW | 0.0005 | 0.2197 | 0.2197 | 0.0002 | 0.0171 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| CTL 21 vs. GO–PEI | 0.639 | 0.0363 | 0.0363 | 0.0002 | 0.0024 | 0.0001 | 0.4829 | 0.4065 | 0.0001 | 0.9999 |
| CTL 21 vs. GO–PEI + Hg | 0.9579 | 0.1049 | 0.1049 | 0.0063 | 0.0272 | 0.0002 | 0.8785 | 0.5988 | 0.0001 | 0.9999 |
| CTL 21 vs. Hg | 0.402 | 0.2774 | 0.2774 | 0.1064 | 0.0001 | 0.0001 | 0.0042 | 0.5498 | 0.0001 | 0.0001 |
| CTL 21 vs. RSW | 0.0001 | 0.022 | 0.022 | 0.1012 | 0.0011 | 0.8038 | 0.7682 | 0.1474 | 0.0001 | 0.9999 |
| GO–PEI vs. GO–PEI + Hg | 0.5827 | 0.0023 | 0.0023 | 0.0065 | 0.0081 | 0.9759 | 0.4029 | 0.1906 | 0.999 | 0.906 |
| GO–PEI vs. Hg | 0.2006 | 0.0492 | 0.0692 | 0.0181 | 0.0186 | 0.0324 | 0.1087 | 0.1784 | 0.0001 | 0.0001 |
| GO–PEI vs. RSW | 0.0001 | 0.0014 | 0.0014 | 0.0001 | 0.0116 | 0.0011 | 0.8561 | 0.951 | 0.2278 | 0.9101 |
| GO–PEI + Hg vs. Hg | 0.4002 | 0.0001 | 0.0001 | 0.8328 | 0.123 | 0.0436 | 0.0045 | 0.8413 | 0.0001 | 0.0001 |
| GO–PEI + Hg vs. RSW | 0.0004 | 0.0478 | 0.0478 | 0.1776 | 0.7441 | 0.0023 | 0.7046 | 0.0001 | 0.227 | 0.9116 |
| Hg vs. RSW | 0.0004 | 0.0001 | 0.0001 | 0.4525 | 0.094 | 0.0001 | 0.2458 | 0.0009 | 0.0001 | 0.0001 |
| F–value | 6.8 | 7.7088 | 22.168 | 13.823 | 10.034 | 71.835 | 56.966 | 13.846 | 47.304 | 39.377 |
| F (DFn, DFd) | 5, 48 | 5, 12 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, F.; Soares, A.M.V.M.; Figueira, E.; Pereira, E.; Marques, P.A.A.P.; Polese, G.; Freitas, R. The Influence of Temperature Increase on the Toxicity of Mercury Remediated Seawater Using the Nanomaterial Graphene Oxide on the Mussel Mytilus galloprovincialis. Nanomaterials 2021, 11, 1978. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11081978
Coppola F, Soares AMVM, Figueira E, Pereira E, Marques PAAP, Polese G, Freitas R. The Influence of Temperature Increase on the Toxicity of Mercury Remediated Seawater Using the Nanomaterial Graphene Oxide on the Mussel Mytilus galloprovincialis. Nanomaterials. 2021; 11(8):1978. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11081978
Chicago/Turabian StyleCoppola, Francesca, Amadeu M. V. M. Soares, Etelvina Figueira, Eduarda Pereira, Paula A. A. P. Marques, Gianluca Polese, and Rosa Freitas. 2021. "The Influence of Temperature Increase on the Toxicity of Mercury Remediated Seawater Using the Nanomaterial Graphene Oxide on the Mussel Mytilus galloprovincialis" Nanomaterials 11, no. 8: 1978. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11081978