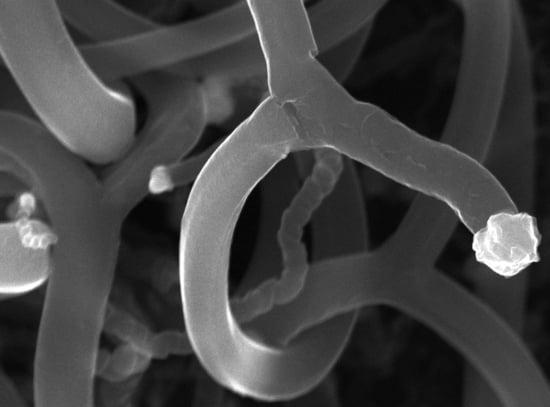
Growth, Properties, and Applications of Branched Carbon Nanostructures
Abstract
:1. Introduction
2. Branching and Growth of Carbon Nanostructures (CNSs)
2.1. Experimental Studies and Branched CNS Production
2.2. Theoretical Studies
3. Applications
3.1. Batteries
| CNS Type | Electrode Type | Specific Capacity | Ref. |
|---|---|---|---|
| Y-type. Nitrogen-doped porous branched MWCNTs (NBNTs) | Co9S8@NBNT 1 nanohybrid anode, Li metal cathode-coin cell | 1109 mA·h·g−1 at 500 mA·g−1 current density (200 cycles) | [45] |
| Tree-like bud-branch composite. VS2 nanosheet “buds” on MWCNT “trees” | VS2 NS@CNTs 1 film on Cu foil current collector as anode working electrode, Li metal foil as counter electrode | ~850 mA·h·g−1 at 200 mA·g−1 current density (100 cycles) | [46] |
| Porous 3D interconnected network of CNS and NiO nanofibers | CNS/NiO coated on Cu foil current collector anode as a working electrode and lithium foil as reference and counter electrode | ~750 mA·h·g−1 at 200 mA·g−1 current density (50 cycles) | [47] |
| Tree-like. MWCNTs with SnO2 branches coated with carbon—“brush-like” structure | b-CNT@SnO2@C 1 Heterostructures as anode | ~800 mA·h·g−1 at 720 mA·g−1 current density (40 cycles) | [44] |
| Tree-like. MWCNT truck with Bi2S3 nanorod branches—“brush-like” structure | Bi2S3-CNT branched hybrid anode with Li metal foil as counter and reference electrode | ~400 mA·h·g−1 at 60 mA·g−1 current density (40 cycles) | [48] |
| Porous carbon hybrids (PAN and MWCNTs)—metal-based nanostructures (MOF) | Co-carbon hybrids (CoCHs) as anodes for rechargeable metal ion batteries | LIB: 680 mA·h·g−1 at 200 mA·g−1 current density (320 cycles) SIB: 220 mA·h·g−1 at 100 mA·g−1 current density (500 cycles) | [49] |
| Core/branch cocoon of MWCNT on sodium manganite nanotubes | SIB cathode material | 158 mA·h·g−1 at 100 mA·g−1 current density (100 cycles) | [53] |
| Tree-like. Graphene foam supported V2O3/MWCNTs core/branch composite arrays | SIB cathode material | 612 mA·h·g−1 at 100 mA·g−1 current density (70 cycles) | [54] |
3.2. Electrocatalysis
| CNS Type | Application | Specifications | Ref. |
|---|---|---|---|
| Biomimetic tree-like, bud-branching | Oxidized MWCNTs with FeOOH “leaves” on Ni foam anode—OER electrocatalyst for water splitting | OER overpotential 210 mV at 10 mA·cm−2. Tafel slope of 31 mV·dec−1 | [87] |
| 3D macroporous. Hierarchical graphene/iron hybrid architectures branched by N-doped MWCNTs | Iron oxide decorated N-doped MWCNTs and iron oxide decorated MWCNTs grown on rGO 1 to form hybrid structures for bifunctional electrocatalysis | ORR onset potential 0.72 V, OER onset potential 1.63 V, Tafel slope of 61 mV·dec−1 | [82] |
| Tree-like hierarchical network | Fe/N-doped MWCNTs with Fe/N-doped b-CNT-ORR electrocatalyst in proton exchange fuel cells (PEMFCs) | ORR onset potential ~0.92 V, Tafel slope of ~60 mV·dec−1 | [83] |
| Tree-like hierarchical architecture | Ni3Co NP catalysis to form N-doped MWCNT branches on CNFs-electrocatalyst for hydrogen production via water splitting | HER overpotential 114 mV at 10 mA·cm−2, Tafel slope of 117 mV·dec−1 | [89] |
| 3D non-aligned hierarchical network | Fe catalyzed growth of primary and secondary branching MWCNTs on a glassy carbon (GC) substrate, then Pt NP electrodeposition to form electrocatalyst | High activity and poisoning stability of the Pt-CNT/CNT/GC electrodes for MeOH oxidation | [96] |
| 3D flower-like structure | N, P co-doped MWCNTs using multi-armed ZIF-8 templating for ORR, OER, and metal-ion batteries | ORR onset potential ~0.75 V, OER onset potential ~1.5 V, Tafel slope of 115 mV·dec−1 | [84] |
| Tree-like, P-doped MWCNT with amorphous MoS2 leaves | Urea-assisted hydrothermal synthesis of tree-like MoS2/MWCNTs composite—HER electrocatalyst | HER overpotential 151 mV at 10 mA cm−2, Tafel slope of 49 mV·dec−1 | [90] |
| Tree-like MWCNTs with MoS2 flake leaves | Leaves-and-branch structure of strongly coupled and porous MoS2-MWCNTs nanocomposite—HER electrocatalyst | HER overpotential ~100 mV at 10 mA·cm−2, Tafel slope of 47 mV·dec−1 | [91] |
| 3D hyperbranched structure | Dendritic hyperbranched HPEK grafted onto the surface of MWCNTs and sulfonated to get water-dispersible SHPEK-g-MWCNT. ORR electrocatalyst. | ORR onset potential ~0.22 V | [85] |
3.3. Supercapacitors
| CNS Type | Material Type | Specifications | Ref. |
|---|---|---|---|
| Tree-like, bud-branch composite | MWCNTs grown on MoO2 NPs decorating Mo-O-C nanorods for pseudocapacitive energy storage | Specific capacitance of 1667 F·g−1 at 1 A·g−1 discharge rate. Rate capability of 93% after 3000 cycles (5 A·g−1) | [103] |
| Tree-like MWCNTs with carbon films | Vertically aligned CNTs on stainless steel substrate for electrochemical capacitor electrodes | Areal capacitance of 0.55 mF·cm−2 (4.6 F·cm−3) at a current density of 0.88 mA·cm−2 (2500 cycles) | [100] |
| Y-type branched MWCNT/CNF | b-CNT/b-CNF composite for supercapacitors | Specific capacitance of ~207 F·g−1 at 1 A·g−1 discharge rate. Rate capability of 96% after 5000 cycles (20 A·g−1) | [101] |
| 3D structure composed of MWCNT tree-like with CNF leaves | Hollow MWCNT/GP micro-conduits composed of MWCNTs with GP branchlets for supercapacitor electrodes | Specific capacitance of 500 F·g−1. Areal capacitance of 2.35 F·cm−2. Rate capability of ~95% after 10,000 cycles | [99] |
| 3D core–shell branched nanostructure CNTs/Ni(OH)2 composites | 3D branched MWCNTs/Ni(OH)2 as a positive electrode for battery–supercapacitor hybrid device (BSH) | MWCNT/Ni(OH)2 cathode-specific capacitance of 1251 F·g−1 at 1 A·g−1. Rate capability of 75% after 2000 cycles | [104] |
3.4. Electromagnetic Wave (EMW) Technology
| CNS Type | Material Type | Specifications | Ref. |
|---|---|---|---|
| 3D tree-like hybrid. VANS (vertically aligned nanostructure) | MWCNTs grown from iron catalysts on black-Si stems, vertically aligned on Si substrate; ultrahigh absorbance at wide spectral range of wavelength | Absorbance of bSi-CNT in the range 300–1200 nm and was 94% at 1200 nm | [105] |
| Flower-like hierarchical nanospheres | Fe catalyzed growth of primary and secondary branching MWCNTs on a glassy carbon substrate; electromagnetic wave absorbers | Reflection loss is −50 dB at a frequency of 7.9 GHz, and efficient absorption bandwidth of 4 GHz | [106] |
| Tree-like. Hollow porous carbon fibers (HPCFs) with MWCNT branches decorated with iron oxide NPs | Fe3O4–CNTs–HPCF absorbents with “tree-like” structure; EM wave absorber. Lightweight composite material for aerospace applications | The bandwidth with a reflection loss <−15 dB from 10 to 18 GHz (1.5–3.0 mm thick layer) and, the minimum reflection loss is −51 dB at 14 GHz (2.5 mm thick layer) | [107] |
3.5. Sensors Technology
| CNS Type | Material Type | Specifications | Ref. |
|---|---|---|---|
| Forked-branched hybrid network CNS | 3D CNS decorated with MWCNTs or carbon black-TPU-based composite piezoresistive strain sensors | Composite 1.5CNS-1CB-gauge factor ~21 (strain ε = 50%) and 99 (strain ε = 100%) | [109] |
| Branched CNS-highly-entangled and wall-sharing MWCNTs | Branched CNSs in TPU matrix as stretchable strain sensors | Electrical percolation threshold (ΦC) of 0.82 wt%. Gauge factors of 15, 30, and 58 for strain levels of 0–44%, 45–73%, and 74–100%, respectively | [110] |
| CNFs with GNP leaves | TPU melt-mixed with MWCNTs and GNPs-dielectric elastomers for shape memory and temperature sensing | An increase in dielectric constant of 10 with low loss tangent, 0.008. Dielectric constant of 13 at RT (1 kHz) | [111] |
| Tree-like MWCNT with CNS leaves | Branched CNS in TPU-highly stretchable strain sensors | Percolation threshold of 0.06 wt% CNS. Composite with 0.7 wt% CNS; electrical conductivity of 1 S/m; gauge factor up to 6861 at strain ε = 660% (elongation at break is 950%) | [108] |
| Y-junction MWCNTs and carbon flakes | TPU/CNS/GNP nanocomposites—melt mix preparation—multifunctional polymer nanocomposites, piezoresistive sensors | At 2 wt% filler concentration: TPU/CNS and TPU/CNS/GNP nanocomposites; gauge factor up to 28 and 144, respectively, under 50% strain | [112] |
| Tree-like MWCNT-COOH/Ag NP with porous structure | MWCNT-COOH/Ag NP nanohybrid CO2 gas detection | Electrochemical CO2 detection in aqueous medium with a detection limit of 52 nM (surface area 525 ms/g) | [113] |
| Hierarchically structured carbon electrodes fabricated from cellulose | MWCNT modified carbon fiber: single fiber microelectrode with branched carbon nanotubes for enhanced sensing | Tested the detection of NADH oxidation. The overpotential of NADH decreased from over 0.8 V to 0.6 V for the CNT-modified carbon fiber electrode | [114] |
3.6. Composite Performance Enhancement
3.7. Other Technological Applications
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenberg, D.; Love, S.; Hubbard, E.; Klimscha, F. 7200 Years Old Constructions and Mudbrick Technology: The Evidence from Tel Tsaf, Jordan Valley, Israel. PLoS ONE 2020, 15, e0227288. [Google Scholar] [CrossRef] [PubMed]
- Masselter, T.; Eckert, S.; Speck, T. Functional Morphology, Biomechanics and Biomimetic Potential of Stem-Branch Connections in Dracaena reflexa and Freycinetia insignis. Beilstein J. Nanotechnol. 2011, 2, 173–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Zhang, L.; Lua, J. Branched Carbon Nanotube Reinforcements for Improved Strength of Polyethylene Nanocomposites. Appl. Phys. Lett. 2012, 101, 161907. [Google Scholar] [CrossRef]
- Slepchenkov, M.M.; Gerasimenko, A.Y.; Telyshev, D.V.; Glukhova, O.E. Protein-Polymer Matrices with Embedded Carbon Nanotubes for Tissue Engineering: Regularities of Formation and Features of Interaction with Cell Membranes. Materials 2019, 12, 3083. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, M.; Rao, G.M. Superhydrophobic Vertically Aligned Treelike Carbon Nanostructures. Phys. Rev. Appl. 2019, 11, 034011. [Google Scholar] [CrossRef]
- Kang, Y.; Yu, X.; Kota, M.; Park, H.S. Carbon Nanotubes Branched on Three-Dimensional, Nitrogen-Incorporated Reduced Graphene Oxide/Iron Oxide Hybrid Architectures for Lithium Ion Battery Anode. J. Alloys Compd. 2017, 726, 88–94. [Google Scholar] [CrossRef]
- Kazemi, K.K.; Bonabi, F.; Shokrzadeh, M. Electric Field-Oriented Growth of Carbon Nanotubes and Y-Branches in a Needle-Pulsed Arc Discharge Unit: Mechanism of the Production. J. Exp. Nanosci. 2015, 10, 1093–1105. [Google Scholar] [CrossRef] [Green Version]
- Santini, C.A.; Vereecken, P.M.; Van Haesendonck, C. Growth of Carbon Nanotube Branches by Electrochemical Decoration of Carbon Nanotubes. Mater. Lett. 2012, 88, 33–35. [Google Scholar] [CrossRef]
- Il’in, O.I.; Il’ina, M.V.; Rudyk, N.N.; Fedotov, A.A. Adhesive Coatings Based on Aligned Arrays of Carbon Nanostructures. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 443, p. 012009. [Google Scholar] [CrossRef]
- Ghosh, M.; Mohan Rao, G. Synthesis of Vertically Aligned and Tree-Like Carbon Nanostructures. Carbon 2018, 133, 239–248. [Google Scholar] [CrossRef]
- He, Z.; Maurice, J.-L.; Chang, S.L.; Cojocaru, C.S.; Pribat, D. Growth Mechanisms of Carbon Nanostructures with Branched Carbon Nanofibers Synthesized by Plasma-Enhanced Chemical Vapour Deposition. CrystEngComm 2014, 16, 2990–2995. [Google Scholar] [CrossRef] [Green Version]
- Romo-Herrera, J.M.; Sumpter, B.G.; Cullen, D.A.; Terrones, H.; Cruz-Silva, E.; Smith, D.J.; Meunier, V.; Terrones, M. An Atomistic Branching Mechanism for Carbon Nanotubes: Sulfur as the Triggering Agent. Angew. Chem. 2008, 47, 2948–2953. [Google Scholar] [CrossRef]
- Wei, Q.; Liu, Y.; Zhang, L.; Huang, S. Growth and Formation Mechanism of Branched Carbon Nanotubes by Pyrolysis of Iron(II) Phthalocyanine. Nano-Micro Lett. 2013, 5, 124–128. [Google Scholar] [CrossRef]
- Shah, T.K.; Malecki, H.C.; Basantkumar, R.R.; Liu, H.; Fleischer, C.A.; Sedlak, J.J.; Patel, J.M.; Burgess, W.P.; Goldfinger, J.M. Carbon Nanostructures and Methods of Making the Same 2014. International Application No. PCT/US2013/062032, 26 September 2013. [Google Scholar]
- Arif, M.F.; Kumar, S.; Shah, T. Tunable Morphology and Its Influence on Electrical, Thermal and Mechanical Properties of Carbon Nanostructure-Buckypaper. Mater. Des. 2016, 101, 236–244. [Google Scholar] [CrossRef]
- Malik, S.; Nemoto, Y.; Guo, H.; Ariga, K.; Hill, J.P. Fabrication and Characterization of Branched Carbon Nanostructures. Beilstein J. Nanotechnol. 2016, 7, 1260–1266. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Park, J.M.; Watanabe, K.; Taniguchi, T.; Jarillo-Herrero, P. Pauli-Limit Violation and Re-entrant Superconductivity in Moiré Graphene. Nature 2021, 595, 526–531. [Google Scholar] [CrossRef]
- Goswami, G.K.; Nandan, R.; Nanda, K.K. Growth of Branched Carbon Nanotubes with Doped/Un-Doped Intratubular Junctions by One-Step Co-Pyrolysis. Carbon 2013, 56, 97–102. [Google Scholar] [CrossRef]
- Li, L.; Liao, X.; Sheng, X.; Hao, Z.; He, L.; Liu, P.; Quan, H.; Zhang, Y. Effect of Structure Regulation of Hyper-Branched Polyester Modified Carbon Nanotubes on Toughening Performance of Epoxy/Carbon Nanotube Nanocomposites. RSC Adv. 2019, 9, 12864–12876. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Xia, L.; Hao, Z.; Sheng, X.; Yi, Z.; Pan, L. Investigation on Cure Kinetics of Epoxy Resin Containing Carbon Nanotubes Modified with Hyper-Branched Polyester. RSC Adv. 2018, 8, 29830–29839. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.H.; Yang, Y.K.; Xie, X.L.; Li, R.K.Y. Dispersion and Crystallization Studies of Hyper-Branched Poly(Urea-Urethane)s-Grafted Carbon Nanotubes Filled Polyamide-6 Nanocomposites. Compos. Part A 2010, 41, 670–677. [Google Scholar] [CrossRef]
- Marchesan, S.; Melchionna, M.; Prato, M. Wire Up on Carbon Nanostructures! How to Play a Winning Game. ACS Nano 2015, 9, 9441–9450. [Google Scholar] [CrossRef] [Green Version]
- Jono, K.; Suzuki, A.; Akita, M.; Albrecht, K.; Yamamoto, K.; Yoshizawa, M. A Polyaromatic Molecular Clip That Enables the Binding of Planar, Tubular, and Dendritic Compounds. Angew. Chem. 2017, 56, 3570–3574. [Google Scholar] [CrossRef]
- Adorinni, S.; Cringoli, M.C.; Perathoner, S.; Fornasiero, P.; Marchesan, S. Green Approaches to Carbon Nanostructure-Based Biomaterials. Appl. Sci. 2021, 11, 2490. [Google Scholar] [CrossRef]
- Iglesias, D.; Senokos, E.; Alemán, B.; Cabana, L.; Navío, C.; Marcilla, R.; Prato, M.; Vilatela, J.J.; Marchesan, S. Gas-Phase Functionalization of Macroscopic Carbon Nanotube Fiber Assemblies: Reaction Control, Electrochemical Properties, and Use for Flexible Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 5760–5770. [Google Scholar] [CrossRef]
- Clement, P.; Trinchera, P.; Cervantes-Salguero, K.; Ye, Q.; Jones, C.R.; Palma, M. A One-Step Chemical Strategy for the Formation of Carbon Nanotube Junctions in Aqueous Solution: Reaction of DNA-Wrapped Carbon Nanotubes with Diazonium Salts. ChemPlusChem 2019, 84, 1235–1238. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Tiwari, R.S. Growth Analysis and High-Yield Synthesis of Aligned-Stacked Branched Nitrogen-Doped Carbon Nanotubes Using Sesame Oil as a Natural Botanical Hydrocarbon Precursor. Mater. Des. 2016, 94, 166–175. [Google Scholar] [CrossRef]
- Zhuo, C.; Levendis, Y.A. Upcycling Waste Plastics into Carbon Nanomaterials: A Review. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Joseph Berkmans, A.; Jagannatham, M.; Priyanka, S.; Haridoss, P. Synthesis of Branched, Nano Channeled, Ultrafine and Nano Carbon Tubes from PET Wastes Using the Arc Discharge Method. Waste Manag. 2014, 34, 2139–2145. [Google Scholar] [CrossRef]
- Zhang, G.; Glukhova, O.E. New Automatic Method for Generating Atomistic Models of Multi-Branched and Arbitrary-Shaped Seamless Junctions of Carbon Nanostructures. Comput. Mater. Sci. 2020, 184, 109943. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Zhong, J.; Liu, Z.; Cheng, G.-G.; Ding, J.-N. Service Performance of Nanopins Based on Branched Carbon Nanotubes. Micro Nano Lett. 2017, 12, 934–939. [Google Scholar] [CrossRef]
- Matsui, K.; Segawa, Y.; Namikawa, T.; Kamada, K.; Itami, K. Synthesis and Properties of All-Benzene Carbon Nanocages: A Junction Unit of Branched Carbon Nanotubes. Chem. Sci. 2013, 4, 84–88. [Google Scholar] [CrossRef]
- Chen, W.-J.; Chang, I.L. The Atomistic Study on Thermal Transport of the Branched CNT. J. Mech. 2020, 36, 721–727. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Prakash, V. Phonon Scattering at SWCNT-SWCNT Junctions in Branched Carbon Nanotube Networks. J. Nanopart. Res. 2015, 17, 1–13. [Google Scholar] [CrossRef]
- Lekshmi, G.; Sana, S.S.; Nguyen, V.H.; Nguyen, T.H.C.; Nguyen, C.C.; Le, Q.V.; Peng, W. Recent Progress in Carbon Nanotube Polymer Composites in Tissue Engineering and Regeneration. Int. J. Mol. Sci. 2020, 21, 6440. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, D.; Bosi, S.; Melchionna, M.; Da Ros, T.; Marchesan, S. The Glitter of Carbon Nanostructures in Hybrid/Composite Hydrogels for Medicinal Use. Curr. Top. Med. Chem. 2016, 16, 1976–1989. [Google Scholar] [CrossRef] [Green Version]
- Aoki, K.; Ogihara, N.; Tanaka, M.; Haniu, H.; Saito, N. Carbon Nanotube-Based Biomaterials for Orthopaedic Applications. J. Mater. Chem. B 2020, 8, 9227–9238. [Google Scholar] [CrossRef]
- Pei, B.; Wang, W.; Dunne, N.; Li, X. Applications of Carbon Nanotubes in Bone Tissue Regeneration and Engineering: Superiority, Concerns, Current Advancements, and Prospects. Nanomaterials 2019, 9, 1501. [Google Scholar] [CrossRef] [Green Version]
- Marchesan, S.; Bosi, S.; Alshatwi, A.; Prato, M. Carbon Nanotubes for Organ Regeneration: An Electrifying performance. Nano Today 2016, 11, 398–401. [Google Scholar] [CrossRef]
- Marchesan, S.; Ballerini, L.; Prato, M. Nanomaterials for Stimulating Nerve Growth. Science 2017, 356, 1010–1011. [Google Scholar] [CrossRef] [Green Version]
- Amin, D.R.; Sink, E.; Narayan, S.; Abdel-Hafiz, M.; Mestroni, L.; Peña, B. Nanomaterials for Cardiac Tissue Engineering. Molecules 2020, 25, 5189. [Google Scholar] [CrossRef]
- Savostyanov, G.V.; Slepchenkov, M.M.; Shmygin, D.S.; Glukhova, O.E. Specific Features Ofstructure, Electrical Conductivity and Interlayer Adhesion of the Natural Polymer Matrix from the Layers of Branched Carbon Nanotube Networks Filled with Albumin, Collagen and Chitosan. Coatings 2018, 8, 378. [Google Scholar] [CrossRef] [Green Version]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The Success Story of Graphite as a Lithium-Ion Anode Material-Fundamentals, Remaining Challenges, and Recent Developments Including Silicon (Oxide) Composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Chen, S.; Xin, Y.; Zhou, Y.; Zhang, F.; Ma, Y.; Zhou, H.; Qi, L. Branched CNT@SnO2 Nanorods@Carbon Hierarchical Heterostructures for Lithium Ion Batteries with High Reversibility and Rate Capability. J. Mater. Chem. A 2014, 2, 15582–15589. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, Y.; Xu, B.; Zhang, X.; Al-Ghanim, K.A.; Mahboob, S. Cobalt Sulfide Confined in N-Doped Porous Branched Carbon Nanotubes for Lithium-Ion Batteries. Nano-Micro Lett. 2019, 11, 29. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Fu, J.; Sun, Y.; Sun, M.; Cheng, S.; Chen, K.; Yang, X.; Lou, Q.; Xu, T.; Shang, Y.; et al. Design and Understanding of Core/Branch-Structured VS2 Nanosheets@CNTs as High-Performance Anode Materials for Lithium-Ion Batteries. Nanoscale 2019, 11, 13343–13353. [Google Scholar] [CrossRef]
- Lalia, B.S.; Khalil, A.; Shah, T.; Hashaikeh, R. Flexible Carbon Nanostructures with Electrospun Nickel Oxide as a Lithium-Ion Battery Anode. Ionics 2015, 21, 2755–2762. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, T.; Xia, H.; Zhang, L.; Jiang, J.; Shen, M.; Ni, J.; Gao, L. Branch-Structured Bi2S3-CNT Hybrids with Improved Lithium Storage Capability. J. Mater. Chem. A 2014, 2, 13854–13858. [Google Scholar] [CrossRef]
- Du, M.; Song, D.; Huang, A.; Chen, R.; Jin, D.; Rui, K.; Zhang, C.; Zhu, J.; Huang, W. Stereoselectively Assembled Metal-Organic Framework (MOF) Host for Catalytic Synthesis of Carbon Hybrids for Alkaline-Metal-Ion Batteries. Angew. Chem. 2019, 58, 5307–5311. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Suh, D.H.; Nakhanivej, P.; Kang, Y.; Park, H.S. Iron Oxide Nanoparticle-Encapsulated CNT Branches Grown on 3D Ozonated CNT Internetworks for Lithium-Ion Battery Anodes. Adv. Funct. Mater. 2018, 28, 1801746. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Y.; Liu, N.; Li, J.; Ouyang, C. Theoretical Prediction of T-Graphene as a Promising Alkali-Ion Battery Anode Offering Ultrahigh Capacity. Phys. Chem. Chem. Phys. 2020, 22, 3281–3289. [Google Scholar] [CrossRef]
- Stevens, D.A.; Dahn, J.R. High Capacity Anode Materials for Rechargeable Sodium-Ion Batteries. J. Electrochem. Soc. 2000, 147, 1271. [Google Scholar] [CrossRef]
- Zhong, Y.; Xia, X.; Zhan, J.; Wang, X.; Tu, J. A CNT Cocoon on Sodium Manganate Nanotubes Forming a Core/Branch Cathode Coupled with a Helical Carbon Nanofibre Anode for Enhanced Sodium Ion Batteries. J. Mater. Chem. A 2016, 4, 11207–11213. [Google Scholar] [CrossRef]
- Xia, X.; Chao, D.; Zhang, Y.; Zhan, J.; Zhong, Y.; Wang, X.; Wang, Y.; Shen, Z.X.; Tu, J.; Fan, H.J. Generic Synthesis of Carbon Nanotube Branches on Metal Oxide Arrays Exhibiting Stable High-Rate and Long-Cycle Sodium-Ion Storage. Small 2016, 12, 3048–3058. [Google Scholar] [CrossRef]
- Sheng, J.; Zhu, S.; Jia, G.; Liu, X.; Li, Y. Carbon Nanotube Supported Bifunctional Electrocatalysts Containing Iron-Nitrogen-Carbon Active Sites for Zinc-Air Batteries. Nano Res. 2021. [Google Scholar] [CrossRef]
- Kweon, D.H.; Okyay, M.S.; Kim, S.-J.; Jeon, J.-P.; Noh, H.-J.; Park, N.; Mahmood, J.; Baek, J.-B. Ruthenium Anchored on Carbon Nanotube Electrocatalyst for Hydrogen Production with Enhanced Faradaic Efficiency. Nat. Commun. 2020, 11, 1278. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Ge, L.; Veksha, A.; Lisak, G. Cobalt and Nitrogen Co-Doped Porous Carbon/Carbon Nanotube Hybrids Anchored with Nickel Nanoparticles as High-Performance Electrocatalysts for Oxygen Reduction Reactions. Nanoscale 2020, 12, 13028–13033. [Google Scholar] [CrossRef]
- Su, S.; Huang, L.; Su, S.; Meng, C.; Zhou, H.; Zhang, L.; Bian, T.; Yuan, A. Fe/Fe3C-NC Nanosheet/Carbon Nanotube Composite Electrocatalysts for Oxygen Reduction Reaction. ACS Appl. Nano Mater. 2020, 3, 11574–11580. [Google Scholar] [CrossRef]
- Li, X.; Ni, L.; Zhou, J.; Xu, L.; Lu, C.; Yang, G.; Ding, W.; Hou, W. Encapsulation of Fe Nanoparticles into an N-Doped Carbon Nanotube/Nanosheet Integrated Hierarchical Architecture as an Efficient and Ultrastable Electrocatalyst for the Oxygen Reduction Reaction. Nanoscale 2020, 12, 13987–13995. [Google Scholar] [CrossRef]
- Majeed, A.; Li, X.; Hou, P.-X.; Tabassum, H.; Zhang, L.; Liu, C.; Cheng, H.-M. Monolayer Carbon-Encapsulated Mo-Doped Ni Nanoparticles Anchored on Single-Wall Carbon Nanotube Film for Total Water Splitting. Appl. Catal. B Environ. 2020, 269, 118823. [Google Scholar] [CrossRef]
- Ali, A.; Shen, P.K. Nonprecious Metal’s Graphene-Supported Electrocatalysts for Hydrogen Evolution Reaction: Fundamentals to Applications. Carbon Energy 2020, 2, 99–121. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-S.; Li, J.-Y.; Huang, M.-J.; Kong, L.-X.; Wu, Z. Anchoring RuxP on 3D Hollow Graphene Nanospheres as Efficient and pH-Universal Electrocatalysts for the Hydrogen Evolution Reaction. Carbon 2020, 161, 44–50. [Google Scholar] [CrossRef]
- Lenarda, A.; Bevilacqua, M.; Tavagnacco, C.; Nasi, L.; Criado, A.; Vizza, F.; Melchionna, M.; Prato, M.; Fornasiero, P. Selective Electrocatalytic H2O2 Generation by Cobalt@N-Doped Graphitic Carbon Core–Shell Nanohybrids. ChemSusChem 2019, 12, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Bracamonte, M.V.; Melchionna, M.; Stopin, A.; Giulani, A.; Tavagnacco, C.; Garcia, Y.; Fornasiero, P.; Bonifazi, D.; Prato, M. Carboxylated, Fe-Filled Multiwalled Carbon Nanotubes as Versatile Catalysts for O2 Reduction and H2 Evolution Reactions at Physiological pH. Chem. Eur. J. 2015, 36, 12769–12777. [Google Scholar] [CrossRef]
- Ruiz-Cornejo, J.C.; Vivo-Vilches, J.F.; Sebastián, D.; Martínez-Huerta, M.V.; Lázaro, M.J. Carbon Nanofiber-Supported Tantalum Oxides as Durable Catalyst for the Oxygen Evolution Reaction in Alkaline Media. Renew. Energy 2021, 178, 307–317. [Google Scholar] [CrossRef]
- Cringoli, M.C.; Perathoner, S.; Fornasiero, P.; Marchesan, S. Carbon Nanostructures Decorated with Titania: Morphological Control and Applications. Appl. Sci. 2021, 11, 6814. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Lu, J.; Yang, B.; San, X.; Wu, Z.-S. 2D Intrinsically Defective RuO2/Graphene Heterostructures as All-pH Efficient Oxygen Evolving Electrocatalysts with Unprecedented Activity. Nano Energy 2020, 78, 105185. [Google Scholar] [CrossRef]
- Valenti, G.; Melchionna, M.; Montini, T.; Boni, A.; Nasi, L.; Fonda, E.; Criado, A.; Zitolo, A.; Voci, S.; Bertoni, G.; et al. Water-Mediated ElectroHydrogenation of CO2 at Near-Equilibrium Potential by Carbon Nanotubes/Cerium Dioxide Nanohybrids. ACS Appl. Energy Mater. 2020, 3, 8509–8518. [Google Scholar] [CrossRef]
- Tsounis, C.; Lu, X.; Bedford, N.M.; Subhash, B.; Thomsen, L.; Zhang, Q.; Ma, Z.; Ostrikov, K.; Bendavid, A.; Scott, J.A.; et al. Valence Alignment of Mixed Ni–Fe Hydroxide Electrocatalysts through Preferential Templating on Graphene Edges for Enhanced Oxygen Evolution. ACS Nano 2020, 14, 11327–11340. [Google Scholar] [CrossRef]
- Rozhin, P.; Melchionna, M.; Fornasiero, P.; Marchesan, S. Nanostructured Ceria: Biomolecular Templates and (Bio) Applications. Nanomaterials 2021, 11, 2259. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, B.; Ding, J.; Xu, N.; Bernards, M.T.; He, Y.; Shi, Y. Three-Dimensional Nitrogen-Doped Graphene Aerogel-Supported MnO Nanoparticles as Efficient Electrocatalysts for CO2 Reduction to CO. ACS Sustain. Chem. Eng. 2020, 8, 4983–4994. [Google Scholar] [CrossRef]
- Kralj, S.; Longobardo, F.; Iglesias, D.; Bevilacqua, M.; Tavagnacco, C.; Criado, A.; Delgado Jaen, J.J.; Makovec, D.; Marchesan, S.; Melchionna, M.; et al. Ex-Solution Synthesis of Sub-5-nm FeOx Nanoparticles on Mesoporous Hollow N,O-Doped Carbon Nanoshells for Electrocatalytic Oxygen Reduction. ACS Appl. Nano Mater. 2019, 2, 6092–6097. [Google Scholar] [CrossRef]
- Pang, B.; Zhang, M.; Zhou, C.; Dong, H.; Ma, S.; Feng, J.; Chen, Y.; Yu, L.; Dong, L. Heterogeneous FeNi3/NiFe2O4 Nanoparticles with Modified Graphene as Electrocatalysts for High Performance Dye-Sensitized Solar Cells. Chem. Eng. J. 2021, 405, 126944. [Google Scholar] [CrossRef]
- Dong, M.; Liu, X.; Jiang, L.; Zhu, Z.; Shu, Y.; Chen, S.; Dou, Y.; Liu, P.; Yin, H.; Zhao, H. Cobalt-doped Mn3O4 Nanocrystals Embedded in Graphene Nanosheets as a High-Performance Bifunctional Oxygen Electrocatalyst for Rechargeable Zn–Air Batteries. Green Energy Environ. 2020, 5, 499–505. [Google Scholar] [CrossRef]
- Srinivas, K.; Chen, Y.; Wang, B.; Yu, B.; Lu, Y.; Su, Z.; Zhang, W.; Yang, D. Metal–Organic Framework-Derived Fe-Doped Ni3Fe/NiFe2O4 Heteronanoparticle-Decorated Carbon Nanotube Network as a Highly Efficient and Durable Bifunctional Electrocatalyst. ACS Appl. Mater. Interfaces 2020, 12, 55782–55794. [Google Scholar] [CrossRef]
- Lenarda, A.; Bellini, M.; Marchionni, A.; Miller, H.A.; Montini, T.; Melchionna, M.; Vizza, F.; Prato, M.; Fornasiero, P. Nanostructured Carbon Supported Pd-Ceria as Anode Catalysts for Anion Exchange Membrane Fuel Cells Fed with Polyalcohols. Inorg. Chim. Acta 2018, 470, 213–220. [Google Scholar] [CrossRef]
- Melchionna, M.; Bracamonte, M.V.; Giuliani, A.; Nasi, L.; Montini, T.; Tavagnacco, C.; Bonchio, M.; Fornasiero, P.; Prato, M. Pd@TiO2/Carbon Nanohorn Electrocatalysts: Reversible CO2 Hydrogenation to Formic Acid. Energy Environ. Sci. 2018, 11, 1571–1580. [Google Scholar] [CrossRef] [Green Version]
- Melchionna, M.; Fornasiero, P.; Prato, M. Into the Carbon: A Matter of Core and Shell in Advanced Electrocatalysis. APL Mater. 2020, 8, 020905. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Melchionna, M.; Medved, M.; Błoński, P.; Steklý, T.; Bakandritsos, A.; Kment, Š.; Zbořil, R.; Otyepka, M.; Fornaserio, P.; et al. Enhanced On-Site Hydrogen Peroxide Electrosynthesis by a Selectively Carboxylated N-Doped Graphene Catalyst. ChemCatChem 2021, 107. [Google Scholar] [CrossRef]
- Monai, M.; Melchionna, M.; Fornasiero, P. From Metal to Metal-Free Catalysts: Routes to Sustainable Chemistry. In Advances in Catalysis; Song, C., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 63, pp. 1–73. [Google Scholar]
- Wang, Y.; Yan, F.; Ma, X.; Zhu, C.; Zhang, X.; Chen, Y. Hierarchically 3D Bifunctional Catalysts Assembled with 1D MoC Core/Branched N-Doped CNT Arrays for Zinc-Air Batteries. Electrochim. Acta 2021, 367, 137522. [Google Scholar] [CrossRef]
- Park, B.J.; Kim, J.; Park, H.S. Bifunctional Electrocatalysts Based on Hierarchical Graphene/Iron Hybrid Architectures Branched by N-Doped CNT. J. Alloys Compd. 2020, 846, 156244. [Google Scholar] [CrossRef]
- Xia, D.; Tang, F.; Yao, X.; Wei, Y.; Cui, Y.; Dou, M.; Gan, L.; Kang, F. Seeded Growth of Branched Iron-Nitrogen-Doped Carbon Nanotubes as a High Performance and Durable Non-Precious Fuel Cell Cathode. Carbon 2020, 162, 300–307. [Google Scholar] [CrossRef]
- Li, Y.; Yan, Z.; Wang, Q.; Ye, H.; Li, M.; Zhu, L.; Cao, X. Ultrathin, Highly Branched Carbon Nanotube Cluster with Outstanding Oxygen Electrocatalytic Performance. Electrochim. Acta 2018, 282, 224–232. [Google Scholar] [CrossRef]
- Sohn, G.-J.; Choi, H.-J.; Jeon, I.-Y.; Chang, D.W.; Dai, L.; Baek, J.-B. Water-Dispersible, Sulfonated Hyperbranched Poly(Ether-Ketone) Grafted Multiwalled Carbon Nanotubes as Oxygen Reduction Catalysts. ACS Nano 2012, 6, 6345–6355. [Google Scholar] [CrossRef]
- Ruvinskiy, P.S.; Bonnefont, A.; Pham-Huu, C.; Savinova, E.R. Using Ordered Carbon Nanomaterials for Shedding Light on the Mechanism of the Cathodic Oxygen Reduction Reaction. Langmuir 2011, 27, 9018–9027. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Q.; Liu, F.; Zhang, W.; Tan, Z.; Zhou, H.; Huang, Z.; Jiao, S.; Kuang, Y. Biomimetic Design of Ultrathin Edge-Riched FeOOH@Carbon Nanotubes as High-Efficiency Electrocatalysts for Water Splitting. Appl. Catal. B 2019, 255, 117755. [Google Scholar] [CrossRef]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Li, T.; Li, S.; Liu, Q.; Yin, J.; Sun, D.; Zhang, M.; Xu, L.; Tang, Y.; Zhang, Y. Immobilization of Ni3Co Nanoparticles into N-Doped Carbon Nanotube/Nanofiber Integrated Hierarchically Branched Architectures toward Efficient Overall Water Splitting. Adv. Sci. 2020, 7, 1902371. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhou, H.; Zhang, W.; Yao, S. Urea-Assisted Synthesis of Amorphous Molybdenum Sulfide on P-Doped Carbon Nanotubes for Enhanced Hydrogen Evolution. J. Mater. Sci. 2018, 53, 8951–8962. [Google Scholar] [CrossRef]
- Huang, H.; Huang, W.; Yang, Z.; Huang, J.; Lin, J.; Liu, W.; Liu, Y. Strongly Coupled MoS2 Nanoflake-Carbon Nanotube Nanocomposite as an Excellent Electrocatalyst for Hydrogen Evolution Reaction. J. Mater. Chem. A 2017, 5, 1558–1566. [Google Scholar] [CrossRef]
- Benck, J.D.; Hellstern, T.R.; Kibsgaard, J.; Chakthranont, P.; Jaramillo, T.F. Catalyzing the Hydrogen Evolution Reaction (HER) with Molybdenum Sulfide Nanomaterials. ACS Catal. 2014, 4, 3957–3971. [Google Scholar] [CrossRef]
- Simari, C.; Potsi, G.; Policicchio, A.; Perrotta, I.; Nicotera, I. Clay-Carbon Nanotubes Hybrid Materials for Nanocomposite Membranes: Advantages of Branched Structure for Proton Transport under Low Humidity Conditions in PEMFCs. J. Phys. Chem. C 2016, 120, 2574–2584. [Google Scholar] [CrossRef]
- Suh, D.H.; Park, S.K.; Nakhanivej, P.; Kim, Y.; Hwang, S.M.; Park, H.S. Hierarchically Structured Graphene-Carbon Nanotube-Cobalt Hybrid Electrocatalyst for Seawater Battery. J. Power Sources 2017, 372, 31–37. [Google Scholar] [CrossRef]
- Park, S.K.; Choi, K.; Lee, S.-H.; Oh, I.-K.; Park, S.; Park, H.S. CNT Branching of Three-Dimensional Steam-Activated Graphene Hybrid Frameworks for Excellent Rate and Cyclic Capabilities to Store Lithium Ions. Carbon 2017, 116, 500–509. [Google Scholar] [CrossRef]
- Wang, P.; Kulp, K.; Bron, M. Hierarchically Structured 3D Carbon Nanotube Electrodes for Electrocatalytic Applications. Beilstein J. Nanotechnol. 2019, 10, 1475–1487. [Google Scholar] [CrossRef] [Green Version]
- Conway, B. Similarities and Differences between Supercapacitors and Batteries for Storing Electrical Energy. In Electrochemical Supercapacitors; Springer: Berlin/Heidelberg, Germany, 1999; pp. 11–31. [Google Scholar]
- Stojek, Z. The Electrical Double Layer and Its Structure. In Electroanalytical Methods; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3–9. [Google Scholar]
- Xiong, G.; He, P.; Lyu, Z.; Chen, T.; Huang, B.; Chen, L.; Fisher, T.S. Bioinspired Leaves-on-Branchlet Hybrid Carbon Nanostructure for Supercapacitors. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, M.; Rao, G.M. Vertically Aligned Tree-Like Carbon Nanostructure as an Electrode of the Electrochemical Capacitor. J. Solid State Electrochem. 2019, 23, 1605–1611. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, X.; Ge, C.; Zhou, W.; Zhu, Y.; Xu, B. Branched Carbon Nanotube/Carbon Nanofiber Composite for Supercapacitor Electrodes. Mater. Lett. 2019, 246, 174–177. [Google Scholar] [CrossRef]
- Frackowiak, E.; Beguin, F. Carbon Materials for the Electrochemical Storage of Energy in Capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Si, H.; Sun, L.; Zhang, Y.; Wu, L.; Zhang, Y.; Zhang, Y. Enhanced Pseudocapacitive Energy Storage Properties of Budding-Branch Like MoO2@C/CNT Nanorods. Dalton Trans. 2020, 49, 1637–1645. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, Y.; An, Y.; Zhang, Q.; Wang, X.; Yang, X.; Hu, Z. Battery-Supercapacitor Hybrid Device Based on Agarics-derived Porous Nitrogen-Doped Carbon and 3D Branched Nanoarchitectures CNTs/Ni(OH)2. J. Phys. Chem. Solids 2018, 119, 126–137. [Google Scholar] [CrossRef]
- Phan, T.L.; Yu, W.J. CVD-Grown Carbon Nanotube Branches on Black Silicon Stems for Ultrahigh Absorbance in Wide Wavelength Range. Sci. Rep. 2020, 10, 3441. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Sun, H.; Yuan, H.; Zhang, S.; Zhang, X.; Zhu, C.; Zhang, X.; Chen, Y. Three-Dimensional Architectures Assembled with Branched Metal Nanoparticle-Encapsulated Nitrogen-Doped Carbon Nanotube Arrays for Absorption of Electromagnetic Wave. J. Alloys Compd. 2020, 821, 153267. [Google Scholar] [CrossRef]
- Qiu, J.; Qiu, T. Fabrication and Microwave Absorption Properties of Magnetite Nanoparticle-Carbon Nanotube-Hollow Carbon Fiber Composites. Carbon 2015, 81, 20–28. [Google Scholar] [CrossRef]
- Sang, Z.; Ke, K.; Manas-Zloczower, I. Interface Design Strategy for the Fabrication of Highly Stretchable Strain Sensors. ACS Appl. Mater. Interfaces 2018, 10, 36483–36492. [Google Scholar] [CrossRef]
- Ke, K.; Sang, Z.; Manas-Zloczower, I. Hybrid Systems of Three-Dimensional Carbon Nanostructures with Low Dimensional Fillers for Piezoresistive Sensors. Polym. Compos. 2020, 41, 468–477. [Google Scholar] [CrossRef]
- Sang, Z.; Ke, K.; Manas-Zloczower, I. Effect of Carbon Nanotube Morphology on Properties in Thermoplastic Elastomer Composites for Strain Sensors. Compos. Part A 2019, 121, 207–212. [Google Scholar] [CrossRef]
- Ke, K.; McMaster, M.; Christopherson, W.; Singer, K.D.; Manas-Zloczower, I. Effects of Branched Carbon Nanotubes and Graphene Nanoplatelets on Dielectric Properties of Thermoplastic Polyurethane at Different Temperatures. Compos. Part B 2019, 166, 673–680. [Google Scholar] [CrossRef]
- Ke, K.; Solouki Bonab, V.; Yuan, D.; Manas-Zloczower, I. Piezoresistive Thermoplastic Polyurethane Nanocomposites with Carbon Nanostructures. Carbon 2018, 139, 52–58. [Google Scholar] [CrossRef]
- Kaur Billing, B.; Agnihotri, P.K.; Singh, N. Fabrication of Branched Nanostructures for CNT@Ag Nano-Hybrids: Application in CO2 Gas Detection. J. Mater. Chem. C 2017, 5, 4226–4235. [Google Scholar] [CrossRef]
- Zhao, X.; Lu, X.; Tze, W.T.Y.; Wang, P. A Single Carbon Fiber Microelectrode with Branching Carbon Nanotubes for Bioelectrochemical Processes. Biosens. Bioelectron. 2010, 25, 2343–2350. [Google Scholar] [CrossRef]
- Solouki Bonab, V.; Maxian, O.; Manas-Zloczower, I. Carbon Nanofiller Networks-A Comparative Study of Networks Formed by Branched versus Linear Carbon Nanotubes in Thermoplastic Polyurethane. Polymer 2019, 175, 227–234. [Google Scholar] [CrossRef]
- Malik, S. Nanotubes from Atlantis: Magnetite in Pumice as a Catalyst for the Growth of Carbon Nanotubes. Polyhedron 2018, 152, 90–93. [Google Scholar] [CrossRef]
- Krause, B.; Barbier, C.; Kunz, K.; Poetschke, P. Comparative Study of Singlewalled, Multiwalled, and Branched Carbon Nanotubes Melt Mixed in Different Thermoplastic Matrices. Polymer 2018, 159, 75–85. [Google Scholar] [CrossRef]
- Lysenkov, E.A.; Haholkina, Z.O.; Lobko, E.V.; Tkalich, M.H.; Klepko, V.V. Influence of Carbon Nanotubes on the Mechanical Properties of Cross-Linked Polyurethanes. Mater. Sci. 2017, 53, 14–21. [Google Scholar] [CrossRef]
- Tang, J.; Li, H.; Yan, S.; Yan, S. In Situ Synthesis, Structure, and Properties of a Dendritic Branched Nano-Thickening Agent for High Temperature Fracturing Fluid. J. Appl. Polym. Sci. 2020, 137, 48446. [Google Scholar] [CrossRef]
- Sansotera, M.; Talaeemashhadi, S.; Gambarotti, C.; Pirola, C.; Longhi, M.; Ortenzi, M.A.; Navarrini, W.; Bianchi, C.L. Comparison of Branched and Linear Perfluoropolyether Chains Functionalization on Hydrophobic, Morphological and Conductive Properties of Multi-Walled Carbon Nanotubes. Nanomaterials 2018, 8, 176. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Wu, T.; Teng, K.; Wang, W.; Shan, M.; Xu, Z.; Lv, H.; Deng, H. Simultaneous Electrospinning and Spraying toward Branch-Like Nanofibrous Membranes Functionalized with Carboxylated MWCNTs for Dye Removal. Mater. Lett. 2016, 166, 26–29. [Google Scholar] [CrossRef]
- Lutz, C.; Bog, U.; Loritz, T.; Syurik, J.; Malik, S.; Kumar, C.N.S.; Kübel, C.; Bruns, M.; Greiner, C.; Hirtz, M.; et al. Locally Controlled Growth of Individual Lambda-Shaped Carbon Nanofibers. Small 2019, 15, 1803944. [Google Scholar] [CrossRef]
- European Green Deal. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 31 August 2021).
- Bhusal, S.; Sihn, S.; Varshney, V.; Roy, A.K. A study on Mechanical Strength and Stability of Partially-Fused Carbon Nanotube Junctions. Carbon Trends 2021, 3, 100039. [Google Scholar] [CrossRef]
- Liang, X.; Chen, M.; Pan, G.; Wu, J.; Xia, X. New Carbon for Electrochemical Energy Storage and Conversion. Funct. Mater. Lett. 2019, 12, 1950049. [Google Scholar] [CrossRef]
- Rao, R.; Pint, C.L.; Islam, A.E.; Weatherup, R.S.; Hofmann, S.; Meshot, E.R.; Wu, F.; Zhou, C.; Dee, N.; Amama, P.B.; et al. Carbon Nanotubes and Related Nanomaterials: Critical Advances and Challenges for Synthesis toward Mainstream Commercial Applications. ACS Nano 2018, 12, 11756–11784. [Google Scholar] [CrossRef] [Green Version]
- Takesue, I.; Haruyama, J.; Kobayashi, N.; Chiashi, S.; Maruyama, S.; Sugai, T.; Shinohara, H. Superconductivity in Entirely End-Bonded Multiwalled Carbon Nanotubes. Phys. Rev. Lett. 2006, 96, 057001. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, S.; Einarsson, E.; Murakami, Y.; Edamura, T. Growth Process of Vertically Aligned Single-Walled Carbon Nanotubes. Chem. Phys. Lett. 2005, 403, 320–323. [Google Scholar] [CrossRef] [Green Version]
- Einarsson, E.; Murakami, Y.; Kadowaki, M.; Maruyama, S. Growth Dynamics of Vertically Aligned Single-Walled Carbon Nanotubes from In Situ Measurements. Carbon 2008, 46, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Duong, H.M.; Yamamoto, N.; Papavassiliou, D.V.; Maruyama, S.; Wardle, B.L. Inter-Carbon Nanotube Contact in Thermal Transport of Controlled-Morphology Polymer Nanocomposites. Nanotechnology 2009, 20, 155702. [Google Scholar] [CrossRef] [Green Version]
- Kroemer, H. Nobel Lecture: Quasielectric Fields and Band Offsets: Teaching Electrons New Tricks. Rev. Mod. Phys. 2001, 73, 783. [Google Scholar] [CrossRef]
- Adorinni, S.; Rozhin, P.; Marchesan, S. Smart Hydrogels Meet Carbon Nanomaterials for New Frontiers in Medicine. Biomedicines 2021, 9, 570. [Google Scholar] [CrossRef]
- Rozhin, P.; Charitidis, C.; Marchesan, S. Self-Assembling Peptides and Carbon Nanomaterials Join Forces for Innovative Biomedical Applications. Molecules 2021, 26, 4084. [Google Scholar] [CrossRef]
- Bonilla-Represa, V.; Abalos-Labruzzi, C.; Herrera-Martinez, M.; Guerrero-Pérez, M.O. Nanomaterials in Dentistry: State of the Art and Future Challenges. Nanomaterials 2020, 10, 1770. [Google Scholar] [CrossRef]
- Gupta, T.K.; Budarapu, P.R.; Chappidi, S.R.; Sudhir, Y.B.; Paggi, M.; Bordas, S.P. Advances in Carbon Based Nanomaterials for Bio-Medical Applications. Curr. Med. Chem. 2019, 26, 6851–6877. [Google Scholar] [CrossRef]
- Shin, S.R.; Li, Y.C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-Based Materials for Tissue Engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Xia, K.; Wang, H.; Liang, X.; Yin, Z.; Zhang, Y. Advanced Carbon for Flexible and Wearable Electronics. Adv. Mater. 2019, 31, e1801072. [Google Scholar] [CrossRef]
- Peng, B.; Zhao, F.; Ping, J.; Ying, Y. Recent Advances in Nanomaterial-Enabled Wearable Sensors: Material Synthesis, Sensor Design, and Personal Health Monitoring. Small 2020, 16, e2002681. [Google Scholar] [CrossRef]
- Ali, H.; Ghosh, S.; Jana, N.R. Fluorescent Carbon Dots as Intracellular Imaging Probes. Wiley Interdiscip. Rev. Nanomed. 2020, 12, e1617. [Google Scholar] [CrossRef]
- Hernández-Rivera, M.; Zaibaq, N.G.; Wilson, L.J. Toward Carbon Nanotube-Based Imaging Agents for the Clinic. Biomaterials 2016, 101, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Loh, K.P.; Ho, D.; Chiu, G.N.C.; Leong, D.T.; Pastorin, G.; Chow, E.K. Clinical Applications of Carbon Nanomaterials in Diagnostics and Therapy. Adv. Mater. 2018, 30, e1802368. [Google Scholar] [CrossRef]
- Wang, W.; Hou, Y.; Martinez, D.; Kurniawan, D.; Chiang, W.H.; Bartolo, P. Carbon Nanomaterials for Electro-Active Structures: A Review. Polymers 2020, 12, 2946. [Google Scholar] [CrossRef]
- Marchesan, S.; Melchionna, M.; Prato, M. Carbon Nanostructures for Nanomedicine: Opportunities and Challenges. Fuller. Nanotub. Carbon Nanostruct. 2014, 22, 190–195. [Google Scholar] [CrossRef]
- Raja, I.S.; Song, S.J.; Kang, M.S.; Lee, Y.B.; Kim, B.; Hong, S.W.; Jeong, S.J.; Lee, J.C.; Han, D.W. Toxicity of Zero- and One-Dimensional Carbon Nanomaterials. Nanomaterials 2019, 9, 1214. [Google Scholar] [CrossRef] [Green Version]
- Fadeel, B.; Kostarelos, K. Grouping All Carbon Nanotubes into a Single Substance Category is Scientifically Unjustified. Nat. Nanotechnol. 2020, 15, 164. [Google Scholar] [CrossRef] [Green Version]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, S.; Marchesan, S. Growth, Properties, and Applications of Branched Carbon Nanostructures. Nanomaterials 2021, 11, 2728. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11102728
Malik S, Marchesan S. Growth, Properties, and Applications of Branched Carbon Nanostructures. Nanomaterials. 2021; 11(10):2728. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11102728
Chicago/Turabian StyleMalik, Sharali, and Silvia Marchesan. 2021. "Growth, Properties, and Applications of Branched Carbon Nanostructures" Nanomaterials 11, no. 10: 2728. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11102728