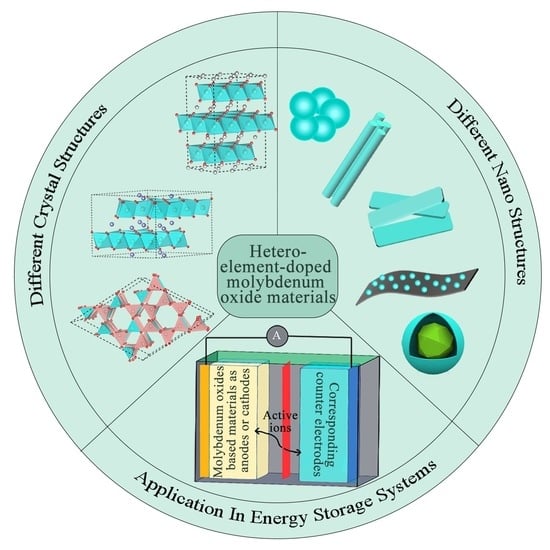
Hetero-Element-Doped Molybdenum Oxide Materials for Energy Storage Systems
Abstract
:1. Introduction
2. Alkali Metal and Alkaline Earth Metal-Doped
2.1. Alkali Metal-Doped
2.2. Alkaline Earth Metal-Doped
3. Transition Metal-Doped
4. Non-Metal-Doped
5. Metal and Non-Metal-Doped Composite
6. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Nai, J.; Zhao, X.; Yuan, H.; Tao, X.; Guo, L. Amorphous carbon-based materials as platform for advanced high-performance anodes in lithium secondary batteries. Nano Res. 2021, 14, 2053–2066. [Google Scholar] [CrossRef]
- Du, H.; Feng, S.; Luo, W.; Zhou, L.; Mai, L. Advanced Li-SexSy battery system: Electrodes and electrolytes. J. Mater. Sci. Technol. 2020, 55, 1–15. [Google Scholar] [CrossRef]
- Manthiram, A. An Outlook on Lithium Ion Battery Technology. ACS Cent. Sci. 2017, 3, 1063–1069. [Google Scholar] [CrossRef] [Green Version]
- Kukushkin, V.Y. Thionyl chloride as comprehensive reagent in preparative inorganic and coordination chemistry. Zhuarnal Neorg. Khimii 1990, 35, 2236–2249. [Google Scholar]
- Hu, X.; Zhang, W.; Liu, X.; Mei, Y.; Huang, Y. Nanostructured Mo-based electrode materials for electrochemical energy storage. Chem. Soc. Rev. 2015, 44, 2376–2404. [Google Scholar] [CrossRef]
- Farsi, H.; Gobal, F.; Raissi, H.; Moghiminia, S. On the pseudocapacitive behavior of nanostructured molybdenum oxide. J. Solid State Electr. 2010, 14, 643–650. [Google Scholar] [CrossRef]
- Zhu, J.; Li, P.; Chen, X.; Legut, D.; Fan, Y.; Zhang, R.; Lu, Y.; Cheng, X.; Zhang, Q. Rational design of graphitic-inorganic Bi-layer artificial SEI for stable lithium metal anode. Energy Storage Mater. 2019, 16, 426–433. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.; Park, Y.; Gwon, H.; Seo, D.; Kim, Y.; Kang, K. SnO2/graphene composite with high lithium storage capability for lithium rechargeable batteries. Nano Res. 2010, 3, 813–821. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Wu, H.B.; Wang, Z.; Lou, X.W.D. Interconnected MoO2 Nanocrystals with Carbon Nanocoating as High-Capacity Anode Materials for Lithium-ion Batteries. ACS Appl. Mater. Inter. 2011, 3, 4853–4857. [Google Scholar] [CrossRef] [PubMed]
- Xuan, H.C.; Zhang, Y.Q.; Xu, Y.K.; Li, H.; Han, P.D.; Wang, D.H.; Du, Y.W. A facile route to large-scale synthesis MoO2 and MoO3 as electrode materials for high-performance supercapacitors. Physic. Status Solidi 2016, 213, 2468–2473. [Google Scholar] [CrossRef]
- Vorobeva, N.S.; Lipatov, A.; Muratov, D.S.; Sinitskii, A. Chemical vapor deposition and characterization of two-dimensional molybdenum dioxide (MoO2) nanoplatelets. Nanotechnology 2018, 29, 505707. [Google Scholar] [CrossRef]
- Zhuang, R.; Yao, S.; Shen, X.; Li, T. Hydrothermal synthesis of mesoporous MoO2 nanospheres as sulfur matrix for lithium sulfur battery. J. Electroanal. Chem. 2019, 833, 441–448. [Google Scholar] [CrossRef]
- Mccrory, M.; Kumar, A.; Ram, M.K. Hydrothermal Synthesis of MoO2 Nanoparticles Directly onto a Copper Substrate. Mrs Adv. 2016, 1, 1051–1054. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, L.; Wu, X.; Lian, L.; Wei, M. Synthesis of MoO2 nanosheets by an ionic liquid route and its electrochemical properties. J. Alloy. Compd. 2013, 580, 358–362. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, C.J.; Lin, S.; Huang, L.; Hu, Z.; Cheng, Y.; Li, T.; Qiao, W.; Long, D.; Huang, Y.; et al. Intercalation of cations into partially reduced molybdenum oxide for high-rate pseudocapacitors. Energy Storage Mater. 2015, 1, 1–8. [Google Scholar] [CrossRef]
- Tomar, A.K.; Marichi, R.B.; Singh, G.; Sharma, R.K. Enhanced electrochemical performance of anion-intercalated lanthanum molybdenum oxide pseudocapacitor electrode. Electrochim. Acta 2019, 296, 120–129. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Duan, X.; Xu, L.; Yang, M.; Yuan, S.; Meng, C.; Wu, Q.; Wang, Q. A novel layered birnessite-type sodium molybdate as dual-ion electrodes for high capacity battery. Electrochim. Acta 2020, 363, 137229. [Google Scholar] [CrossRef]
- Ben-Kamel, K.; Amdouni, N.; Groult, H.; Mauger, A.; Zaghib, K.; Julien, C.M. Structural and electrochemical properties of LiMoO2. J. Power Sources 2012, 202, 314–321. [Google Scholar] [CrossRef]
- Kobayashi, H.; Tabuchi, M.; Shikano, M.; Nishimura, Y.; Kageyama, H.; Ishida, T.; Nakamura, H.; Kurioka, Y.; Kanno, R. Synthesis and electrochemical properties of lithium molybdenum oxides. J. Power Sources 1999, 81, 524–529. [Google Scholar] [CrossRef]
- Komaba, S. Molybdenum oxides synthesized by hydrothermal treatment of A2MoO4 (A=Li, Na, K) and electrochemical lithium intercalation into the oxides. Solid State Ion. 2002, 152–153, 319–326. [Google Scholar] [CrossRef]
- Hibble, S.J.; Fawcett, I.D.; Hannon, A.C. The True Structure and Metal-Metal-Bonded Framework of LiMoIIIO2 Determined from Total Neutron Scattering. Inorg. Chem. 1997, 36, 1749–1753. [Google Scholar] [CrossRef]
- Leroux, F.; Nazar, L.F. Uptake of lithium by layered molybdenum oxide and its tin exchanged derivatives: High volumetric capacity materials. Solid State Ion. 2000, 133, 37–50. [Google Scholar] [CrossRef]
- Verma, R.; Park, C.; Kothandaraman, R.; Varadaraju, U.V. Ternary lithium molybdenum oxide, Li2Mo4O13: A new potential anode material for high-performance rechargeable lithium-ion batteries. Electrochim. Acta 2017, 258, 1445–1452. [Google Scholar] [CrossRef]
- Mikhailova, D.; Bramnik, N.N.; Bramnik, K.G.; Reichel, P.; Oswald, S.; Senyshyn, A.; Trots, D.M.; Ehrenberg, H. Layered LixMoO2 Phases with Different Composition for Electrochemical Application: Structural Considerations. Chem. Mater. 2011, 23, 3429–3441. [Google Scholar] [CrossRef]
- James, A.; Goodenough, J.B. Structure and bonding in Li2MoO3 and Li2-XMoO3 (0 ≤ x ≤ 1.7). J. Solid State Chem. 1988, 76, 87–96. [Google Scholar] [CrossRef]
- Lee, J.; Urban, A.; Li, X.; Su, D.; Hautier, G.; Ceder, G. Unlocking the Potential of Cation-Disordered Oxides for Rechargeable Lithium Batteries. Science 2014, 343, 519–522. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, Y.; Gao, Y.; Yu, X.; Kong, Q.; Gu, L.; Wang, Z.; Yang, X.; Chen, L. Feasibility of Using Li2MoO3 in Constructing Li-Rich High Energy Density Cathode Materials. Chem. Mater. 2014, 26, 3256–3262. [Google Scholar] [CrossRef]
- Liu, X.; Lyu, Y.; Zhang, Z.; Li, H.; Hu, Y.S.; Wang, Z.; Zhao, Y.; Kuang, Q.; Dong, Y.; Liang, Z.; et al. Nanotube Li2MoO4: A novel and high-capacity material as a lithium-ion battery anode. Nanoscale 2014, 6, 13660–13667. [Google Scholar] [CrossRef]
- Mikhailova, D.; Voss, A.; Oswald, S.; Tsirlin, A.A.; Schmidt, M.; Senyshyn, A.; Eckert, J.; Ehrenberg, H. Lithium Insertion into Li2MoO4: Reversible Formation of Li3MoO4 with a Disordered Rock-Salt Structure. Chem. Mater. 2015, 27, 4485–4492. [Google Scholar] [CrossRef]
- Ma, J.; Gao, Y.; Wang, Z.; Chen, L. Structural and electrochemical stability of Li-rich layer structured Li2MoO3 in air. J. Power Sources 2014, 258, 314–320. [Google Scholar] [CrossRef]
- You, J.; Xin, L.; Yu, X.; Zhou, X.; Liu, Y. Synthesis of homogeneous CaMoO4 microspheres with nanopits for high-capacity anode material in Li-ion battery. Appl. Phys. A 2018, 124, 1–8. [Google Scholar] [CrossRef]
- Zhu, K.; Guo, S.; Yi, J.; Bai, S.; Wei, Y.; Chen, G.; Zhou, H. A new layered sodium molybdenum oxide anode for full intercalation-type sodium-ion batteries. J. Mater. Chem. A 2015, 3, 22012–22016. [Google Scholar] [CrossRef]
- Yu, S.; Peng, C.; Li, Z.; Zhang, L.; Xiao, Q.; Lei, G.; Ding, Y. K-Doped Li-Rich Molybdenum-Based Oxide with Improved Electrochemical Properties for Lithium-Ion Batteries. Arab. J. Sci. Eng. 2017, 42, 4291–4298. [Google Scholar] [CrossRef]
- Serdtsev, A.V.; Suetin, D.V.; Solodovnikov, S.F.; Gulyaeva, O.A.; Medvedeva, N.I. Electronic structure and sodium-ion diffusion in glaserite-type A3−xNa1+x(MoO4)2 (A = Cs, K) studied with first-principles calculations. Solid State Ion. 2020, 357, 115484. [Google Scholar] [CrossRef]
- Arroyo-De, D.M.; Krich, C.; Nava-Avendano, J.; Palacin, M.R.; Barde, F. In quest of cathode materials for Ca ion batteries: The CaMO3 perovskites (M = Mo, Cr, Mn, Fe, Co, and Ni). Phys. Chem. Chem. Phys. 2016, 18, 19966–19972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, N.; Shaju, K.M.; Subba Rao, G.V.; Chowdari, B.V.R.; Dong, Z.L.; White, T.J. Carbon-Coated Nanophase CaMoO4 as Anode Material for Li Ion Batteries. Chem. Mater. 2004, 16, 504–512. [Google Scholar] [CrossRef]
- Bullard, J. Structural evolution of the MoO3(010) surface during lithium intercalation. Solid State Ion. 2003, 160, 335–349. [Google Scholar] [CrossRef]
- Mikhailik, V.B.; Kraus, H.; Itoh, M.; Iri, D.; Uchida, M. Radiative decay of self-trapped excitons in CaMoO4 and MgMoO4 crystals. J. Phys. Condens. Matter 2005, 17, 7209–7218. [Google Scholar] [CrossRef]
- Sleight, A.W.; Chamberland, B.L. Transition metal molybdates of the type AMoO4. Inorg. Chem. 1968, 7, 1672–1675. [Google Scholar] [CrossRef]
- Haetge, J.; Suchomski, C.; Brezesinski, T. Ordered Mesoporous β-MgMoO4 Thin Films for Lithium-Ion Battery Applications. Small 2013, 9, 2541–2544. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Reddy, M.V.; Krishnamoorthi, C.; Tripathy, S.; Mahendiran, R.; Rao, G.V.S.; Chowdari, B.V.R. Carbothermal synthesis, spectral and magnetic characterization and Li-cyclability of the Mo-cluster compounds, LiYMo3O8 and Mn2Mo3O8. Electrochim. Acta 2009, 54, 3360–3373. [Google Scholar] [CrossRef]
- Buhrmester, T.; Leyzerovich, N.N.; Bramnik, K.G.; Ehrenberg, H.; Fuess, H. Electrochemical intercalation of lithium in ternary metal molybdates MMoO4 (M=Cu, Zn). Mat. Res. Soc. Symp. Proc. 2003, 756, 671–676. [Google Scholar] [CrossRef]
- Kim, S.; Ogura, S.; Ikuta, H.; Uchimoto, Y.; Wakihara, M. Synthesis of MnMoO4 as High Capacity Anode Material for Li Secondary Battery. Chem. Lett. 2001, 30, 760–761. [Google Scholar] [CrossRef]
- Minakshi, M.; Barmi, M.; Mitchell, D.R.G.; Barlow, A.J.; Fichtner, M. Effect of oxidizer in the synthesis of NiO anchored nanostructure nickel molybdate for sodium-ion battery. Mater. Today Energy 2018, 10, 1–14. [Google Scholar] [CrossRef]
- Cao, H.; Wu, N.; Liu, Y.; Wang, S.; Du, W.; Liu, J. Facile synthesis of rod-like manganese molybdate crystallines with two-dimentional nanoflakes for supercapacitor application. Electrochim. Acta 2017, 225, 605–613. [Google Scholar] [CrossRef]
- Fei, J.; Sun, Q.; Li, J.; Cui, Y.; Huang, J.; Hui, W.; Hu, H. Synthesis and electrochemical performance of α-ZnMoO4 nanoparticles as anode material for lithium ion batteries. Mater. Lett. 2017, 198, 4–7. [Google Scholar] [CrossRef]
- Zhang, L.; He, W.; Liu, Y.; Ling, M.; Zheng, P.; Guo, S. 3D hierarchical flower of copper molybdate Cu3Mo2O9: Synthesis, nanostructure and lithium storage properties. J. Alloy. Compd. 2017, 723, 512–519. [Google Scholar] [CrossRef]
- Zhang, L.; Song, Y.; Wu, W.; Bradley, R.; Hu, Y.; Liu, Y.; Guo, S. Fe2Mo3O8 nanoparticles self-assembling 3D mesoporous hollow spheres toward superior lithium storage properties. Front. Chem. Sci. Eng. 2021, 15, 156–163. [Google Scholar] [CrossRef]
- Das, B.; Reddy, M.V.; Subba Rao, G.V.; Chowdari, B.V.R. Synthesis of Mo-cluster compound, LiHoMo3O8 by carbothermal reduction and its reactivity towards Li. J. Solid State Electr. 2008, 12, 953–959. [Google Scholar] [CrossRef]
- McCarroll, W.H.; Katz, L.; Ward, R. Some Ternary Oxides of Tetravalent Molybdenum. J. Am. Chem. Soc 1957, 5410. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Luo, W.; Huang, Y. Hierarchical self-assembly of Mn2Mo3O8-graphene nanostructures and their enhanced lithium-storage properties. J. Mater. Chem. 2011, 21, 17229. [Google Scholar] [CrossRef]
- Chu, Y.; Shi, X.; Wang, Y.; Fang, Z.; Deng, Y.; Liu, Z.; Dong, Q.; Hao, Z. High temperature solid-state synthesis of dopant-free Fe2Mo3O8 for lithium-ion batteries. Inorg. Chem. Commun. 2019, 107, 107477. [Google Scholar] [CrossRef]
- Guan, B.; Sun, W.; Wang, Y. Carbon-Coated MnMoO4 Nanorod for High-Performance Lithium-Ion Batteries. Electrochim. Acta 2016, 190, 354–359. [Google Scholar] [CrossRef]
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B. The role of graphene for electrochemical energy storage. Nat. Mater. 2015, 14, 271–279. [Google Scholar] [CrossRef]
- Wang, B.; Xin, H.; Li, X.; Cheng, J.; Yang, G.; Nie, F. Mesoporous CNT@TiO2-C Nanocable with Extremely Durable High Rate Capability for Lithium-Ion Battery Anodes. Sci. Rep. 2015, 4, 3729. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.W.; Yabuuchi, N.; Gallant, B.M.; Chen, S.; Kim, B.; Hammond, P.T.; Shao-Horn, Y. High-power lithium batteries from functionalized carbon-nanotube electrodes. Nat. Nanotechnol. 2010, 5, 531–537. [Google Scholar] [CrossRef]
- Fang, B.; Kim, J.H.; Kim, M.; Yu, J. Hierarchical Nanostructured Carbons with Meso–Macroporosity: Design, Characterization, and Applications. Accounts Chem. Res. 2013, 46, 1397–1406. [Google Scholar] [CrossRef]
- Song, H.; Li, Y.; Shang, L.; Tang, Z.; Zhang, T.; Lu, S. Designed controllable nitrogen-doped carbon-dots-loaded MoP nanoparticles for boosting hydrogen evolution reaction in alkaline medium. Nano Energy 2020, 72, 104730. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, Y.; Peng, Z.; Song, H.; Liu, Z.; Liu, B.; Li, B.; Yang, B.; Lu, S. Cobalt-Ruthenium Nanoalloys Parceled in Porous Nitrogen-Doped Graphene as Highly Efficient Difunctional Catalysts for Hydrogen Evolution Reaction and Hydrolysis of Ammonia Borane. ACS Sustain. Chem. Eng. 2019, 7, 7014–7023. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Z.; Chen, J.; Cheng, L.; Chang, J.; Sheng, W.; Hu, C.; Cao, S. C-doped hollow TiO2 spheres: In situ synthesis, controlled shell thickness, and superior visible-light photocatalytic activity. Appl. Catal. B Environ. 2015, 165, 715–722. [Google Scholar] [CrossRef]
- Pan, L.; Muhammad, T.; Ma, L.; Huang, Z.; Wang, S.; Wang, L.; Zou, J.; Zhang, X. MOF-derived C-doped ZnO prepared via a two-step calcination for efficient photocatalysis. Appl. Catal. B Environ. 2016, 189, 181–191. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, Y.; Wang, J.; Sun, L.; Cao, M. Facile fabrication of molybdenum dioxide/nitrogen-doped graphene hybrid as high performance anode material for lithium ion batteries. J. Power Sources 2015, 274, 142–148. [Google Scholar] [CrossRef]
- Fleischmann, S.; Zeiger, M.; Quade, A.; Kruth, A.; Presser, V. Atomic Layer-Deposited Molybdenum Oxide/Carbon Nanotube Hybrid Electrodes: The Influence of Crystal Structure on Lithium-Ion Capacitor Performance. ACS Appl. Mater. Inter. 2018, 10, 18675–18684. [Google Scholar] [CrossRef]
- Yang, X.; Li, Q.; Wang, H.; Feng, J.; Zhang, M.; Yuan, R.; Chai, Y. In-situ carbonization for template-free synthesis of MoO2-Mo2C-C microspheres as high-performance lithium battery anode. Chem. Eng. J. 2018, 337, 74–81. [Google Scholar] [CrossRef]
- Naresh, N.; Jena, P.; Satyanarayana, N. Facile synthesis of MoO3/rGO nanocomposite as anode materials for high performance lithium-ion battery applications. J. Alloy. Compd. 2019, 810, 151920. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C.; Chen, X.; Wang, X.; Li, X.; Yamauchi, Y.; Xu, X.; Wang, J.; Lin, C.; Luo, D.; et al. MoOx nanoparticles anchored on N-doped porous carbon as Li-ion battery electrode. Chem. Eng. J. 2020, 381, 122588. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, X.; Peng, C.; Lei, G.; Li, Z. Pre-intercalation of potassium to improve the electrochemical performance of carbon-coated MoO3 cathode materials for lithium batteries. J. Alloy. Compd. 2020, 826, 154055. [Google Scholar] [CrossRef]
- Xia, Q.; Zhao, H.; Du, Z.; Zeng, Z.; Gao, C.; Zhang, Z.; Du, X.; Kulka, A.; Świerczek, K. Facile synthesis of MoO3/carbon nanobelts as high-performance anode material for lithium ion batteries. Electrochim. Acta 2015, 180, 947–956. [Google Scholar] [CrossRef]
- Noerochim, L.; Wang, J.; Wexler, D.; Chao, Z.; Liu, H. Rapid synthesis of free-standing MoO3/Graphene films by the microwave hydrothermal method as cathode for bendable lithium batteries. J. Power Sources 2013, 228, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Park, G.D.; Hong, J.H.; Lee, J.; Kang, Y.C. Yolk–shell-structured microspheres composed of N-doped-carbon-coated NiMoO4 hollow nanospheres as superior performance anode materials for lithium-ion batteries. Nanoscale 2019, 11, 631–638. [Google Scholar] [CrossRef]
- Ahn, J.H.; Park, G.D.; Kang, Y.C.; Lee, J. Phase-pure β-NiMoO4 yolk-shell spheres for high-performance anode materials in lithium-ion batteries. Electrochim. Acta 2015, 174, 102–110. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, W.; Zhou, C.; Yang, L.; Wang, H.; Gao, Q.; Zhu, M. N-doped carbon encapsulated CoMoO4 nanorods as long-cycle life anode for sodium-ion batteries. J. Colloid Interf. Sci. 2020, 576, 176–185. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, A.J.; Arshad, M.; Javed, M.S.; Ahmad, A.; Shah, S.S.A.; Khan, M.R.; Akram, S.; Zulfiqar; Ali, S.; et al. Charge storage in binder-free 2D-hexagonal CoMoO4 nanosheets as a redox active material for pseudocapacitors. Ceram. Int. 2021, 47, 8659–8667. [Google Scholar] [CrossRef]
- Zhang, J.; Li, R.; Chen, Q.; Zhao, G.; Jia, J. Porous carbon-coated Li2MoO4 as high-performance anode materials for lithium-ion batteries. Mater. Lett. 2018, 233, 302–305. [Google Scholar] [CrossRef]
- Mai, L.Q.; Hu, B.; Chen, W.; Qi, Y.Y.; Lao, C.S.; Yang, R.S.; Dai, Y.; Wang, Z.L. Lithiated MoO3 Nanobelts with Greatly Improved Performance for Lithium Batteries. Adv. Mater. 2007, 19, 3712–3716. [Google Scholar] [CrossRef]
- Qiu, J.; Yang, Z.; Li, Y. N-doped carbon encapsulated ultrathin MoO3 nanosheets as superior anodes with high capacity and excellent rate capability for Li-ion batteries. J. Mater. Chem. A 2015, 3, 24245–24253. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, X.; Liu, J.; Li, S.; Wang, H.; Yang, L.; Liu, S.; Xie, T. Novel Amorphous MoS2/MoO3/Nitrogen-Doped Carbon Composite with Excellent Electrochemical Performance for Lithium Ion Batteries and Sodium Ion Batteries. ACS Sustain. Chem. Eng. 2017, 5, 8025–8034. [Google Scholar] [CrossRef]
- Xiu, Z.; Kim, D.; Alfaruqi, M.H.; Song, J.; Kim, S.; Duong, P.T.; Mathew, V.; Baboo, J.P.; Kim, J. Ultrafine molybdenum oxycarbide nanoparticles embedded in N-doped carbon as a superior anode material for lithium-ion batteries. J. Alloy. Compd. 2017, 696, 143–149. [Google Scholar] [CrossRef]
- Mai, L.Q.; Yang, F.; Zhao, Y.L.; Xu, X.; Xu, L.; Luo, Y.Z. Hierarchical MnMoO4/CoMoO4 heterostructured nanowires with enhanced supercapacitor performance. Nature Commun. 2011, 2, 1–5. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Z.; Fu, H.; Xiao, R.; Fu, M.; Yue, H.; Huang, J. Swallow-Nest Architectures with Cobalt Molybdate Particulates Fixed Constructed Carbon Nanotube Supports for Stable Sodium-Ion Battery Anode. J. Electrochem. Soc. 2020, 167, 80528. [Google Scholar] [CrossRef]
- Xue, R.; Hong, W.; Pan, Z.; Jin, W.; Zhao, H.; Song, Y.; Zhou, J.; Liu, Y. Enhanced electrochemical performance of ZnMoO4/reduced graphene oxide composites as anode materials for lithium-ion batteries. Electrochim. Acta 2016, 222, 838–844. [Google Scholar] [CrossRef] [Green Version]





| Active Component | Application Fields | Specific Capacity (mA h g−1) | Cycles | Current Density (mA g−1) | Voltage Window (V) | Refs. | |
|---|---|---|---|---|---|---|---|
| Alkali metal | Na1/3MoO2·H2O | Li/Na dual-ion battery (cathode) | 650 | 500 | 50 | 0~3.0 | [18] |
| Li2MoO4@C | Li-ion battery (anode) | 504 | 150 | 100 | 0.02~3.0 | [75] | |
| Li2Mo4O13 | Li-ion battery (anode) | 768 | 50 | 106 | 0.1~2.5 | [24] | |
| Lithiated MoO3 Nanobelts | Li-ion battery (cathode) | 220 | 15 | 50 | 0.15~3.0 | [76] | |
| Non-metal | 3D-MoOx@CN-700 | Li-ion battery (anode) | 431 | 1000 | 1000 | 0.01~3.0 | [66] |
| MoO3/NC | Li-ion battery (anode) | 1250 | 60 | 410 | 0.01~3.0 | [77] | |
| a-MM/NCc | Sodium-ion battery (anode) | 1253 | 50 | 100 | 0.01~3.0 | [78] | |
| MoOC/N-doped C | Li-ion battery (anode) | 793 | 100 | 100 | 0.01~3.0 | [79] | |
| Transition metal or doped non-metal composites | MnMoO4@C | Li-ion battery (anode) | 1050 | 200 | 100 | 0~3.0 | [54] |
| NiMoO4 | Sodium-ion battery (anode) | 245 | 100 | 50 | 0~3.0 | [45] | |
| Cu3Mo2O9 | Li-ion battery (anode) | 632 | 200 | 100 | 0.01~3.0 | [53] | |
| MnMoO4/CoMoO4 | Supercapacitor | 187 (F g−1) | 1000 | 1000 | 0.1~0.4 | [80] | |
| CoMoO4/AF-CNT | Sodium-ion battery (anode) | 220 | 200 | 100 | 0.01~3.0 | [81] | |
| ZnMoO4/rGO | Li-ion battery (anode) | 632 | 100 | 100 | 0.01~3.0 | [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, B.; Jian, S.; Yin, G.; Feng, W.; Cao, Y.; Bai, J.; Lai, Y.; Tan, H.; Dong, Y. Hetero-Element-Doped Molybdenum Oxide Materials for Energy Storage Systems. Nanomaterials 2021, 11, 3302. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123302
Hu B, Jian S, Yin G, Feng W, Cao Y, Bai J, Lai Y, Tan H, Dong Y. Hetero-Element-Doped Molybdenum Oxide Materials for Energy Storage Systems. Nanomaterials. 2021; 11(12):3302. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123302
Chicago/Turabian StyleHu, Bo, Shuofeng Jian, Ge Yin, Wenhao Feng, Yaowen Cao, Jiaxuan Bai, Yanan Lai, Huiyun Tan, and Yifan Dong. 2021. "Hetero-Element-Doped Molybdenum Oxide Materials for Energy Storage Systems" Nanomaterials 11, no. 12: 3302. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123302