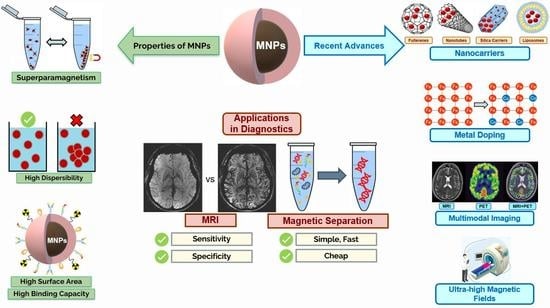
A Comprehensive Updated Review on Magnetic Nanoparticles in Diagnostics
Abstract
:1. Introduction
2. Materials and Methods
3. Magnetic Nanoparticles
3.1. Main Properties/Characteristics
3.2. Application of MNPs in Medicine
3.2.1. Imaging
3.2.2. Molecular Diagnosis
Nucleic Acid Separation and Detection
Protein Purification
Cell Separation
4. Iron Oxide MNPs
4.1. Properties of IONPs
4.2. Synthesis of IONPs
4.3. Clinically Approved IONPs
4.4. IONPs in Clinical Trials
4.5. Limitations of IONPs
5. Other Inorganic MNPs
5.1. Gadolinium
5.2. Cobalt
5.3. Manganese
5.4. Dysprosium
5.5. Holmium
5.6. Other Lanthanides
5.7. Silica
5.8. Copper
5.9. Metal Alloy MNPs
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Richards, M.A. The National Awareness and Early Diagnosis Initiative in England: Assembling the evidence. Br. J. Cancer 2009, 101, S1–S4. [Google Scholar] [CrossRef]
- Virnig, B.A.; Baxter, N.N.; Habermann, E.B.; Feldman, R.D.; Bradley, C.J. A matter of race: Early-versus late-stage cancer diagnosis. Health Aff. 2009, 28, 160–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lushniak, B.D. Surgeon general’s perspectives. Public Health Rep. 2014, 129, 314–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorpe, K.E.; Joski, P.; Johnston, K.J. Antibiotic-resistant infection treatment costs have doubled since 2002, now exceeding $2 billion annually. Health Aff. 2018, 37, 662–669. [Google Scholar] [CrossRef] [PubMed]
- McCormack, V.; Aggarwal, A. Early cancer diagnosis: Reaching targets across whole populations amidst setbacks. Br. J. Cancer 2021, 124, 1181–1182. [Google Scholar] [CrossRef]
- Pellico, J.; Ellis, C.M.; Davis, J.J. Nanoparticle-Based Paramagnetic Contrast Agents for Magnetic Resonance Imaging. Contrast Media Mol. Imaging 2019, 2019, 1845637. [Google Scholar] [CrossRef]
- Tang, C.; He, Z.; Liu, H.; Xu, Y.; Huang, H.; Yang, G.; Xiao, Z.; Li, S.; Liu, H.; Deng, Y.; et al. Application of magnetic nanoparticles in nucleic acid detection. J. Nanobiotechnology 2020, 18, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crist, R.M.; Dasa, S.S.K.; Liu, C.H.; Clogston, J.D.; Dobrovolskaia, M.A.; Stern, S.T. Challenges in the development of nanoparticle-based imaging agents: Characterization and biology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1665. [Google Scholar] [CrossRef]
- Martinelli, C.; Pucci, C.; Ciofani, G. Nanostructured carriers as innovative tools for cancer diagnosis and therapy. APL Bioeng. 2019, 3. [Google Scholar] [CrossRef] [Green Version]
- Kaur, P.; Aliru, M.L.; Chadha, A.S.; Asea, A.; Krishnan, S. Hyperthermia using nanoparticles—Promises and Pitfalls. Int. J. Hyperth. 2016, 32, 76–88. [Google Scholar] [CrossRef]
- Boulaiz, H.; Alvarez, P.J.; Ramirez, A.; Marchal, J.A.; Prados, J.; Rodríguez-Serrano, F.; Perán, M.; Melguizo, C.; Aranega, A. Nanomedicine: Application areas and development prospects. Int. J. Mol. Sci. 2011, 12, 3303–3321. [Google Scholar] [CrossRef] [Green Version]
- Boverhof, D.R.; Bramante, C.M.; Butala, J.H.; Clancy, S.F.; Lafranconi, W.M.; West, J.; Gordon, S.C. Comparative assessment of nanomaterial definitions and safety evaluation considerations. Regul. Toxicol. Pharmacol. 2015, 73, 137–150. [Google Scholar] [CrossRef] [Green Version]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, properties, and regulatory issues. Front. Chem. 2018, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, J.W.; Cheon, J. Magnetic nanoparticles for multi-imaging and drug delivery. Mol. Cells 2013, 35, 274–284. [Google Scholar] [CrossRef]
- Huang, G.; Li, H.; Chen, J.; Zhao, Z.; Yang, L.; Chi, X.; Chen, Z.; Wang, X.; Gao, J. Tunable T1 and T2 contrast abilities of manganese-engineered iron oxide nanoparticles through size control. Nanoscale 2014, 6, 10404–10412. [Google Scholar] [CrossRef]
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutiérrez, L.; Morales, M.P.; Böhm, I.B.; Heverhagen, J.T.; Prosperi, D.; Parak, W.J. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334. [Google Scholar] [CrossRef]
- Hosu, O.; Tertis, M.; Cristea, C. Implication of Magnetic Nanoparticles in Cancer Detection, Screening and Treatment. Magnetochemistry 2019, 5, 55. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, V.; Ganesan, R.; Abdulrahman Syedahamed, H.H.; Thaiyan, M. Effect of cobalt doping on structural, optical, and magnetic properties of ZnO nanoparticles synthesized by coprecipitation method. J. Phys. Chem. C 2014, 118, 9717–9725. [Google Scholar] [CrossRef]
- de Mello, L.B.; Varanda, L.C.; Sigoli, F.A.; Mazali, I.O. Co-precipitation synthesis of (Zn-Mn)-co-doped magnetite nanoparticles and their application in magnetic hyperthermia. J. Alloys Compd. 2019, 779, 698–705. [Google Scholar] [CrossRef]
- Pardo, A.; Pelaz, B.; Gallo, J.; Bañobre-López, M.; Parak, W.J.; Barbosa, S.; Del Pino, P.; Taboada, P. Synthesis, Characterization, and Evaluation of Superparamagnetic Doped Ferrites as Potential Therapeutic Nanotools. Chem. Mater. 2020, 32, 2220–2231. [Google Scholar] [CrossRef]
- Al-Rawi, N.N.; Anwer, B.A.; Al-Rawi, N.H.; Uthman, A.T.; Ahmed, I.S. Magnetism in drug delivery: The marvels of iron oxides and substituted ferrites nanoparticles. Saudi Pharm. J. 2020, 28, 876–887. [Google Scholar] [CrossRef]
- Pelaz, B.; Charron, G.; Pfeiffer, C.; Zhao, Y.; De La Fuente, J.M.; Liang, X.J.; Parak, W.J.; Del Pino, P. Interfacing engineered nanoparticles with biological systems: Anticipating adverse nano-bio interactions. Small 2013, 9, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Zhang, Q.; Lai, W.; Yin, T.; Zhang, C.; Yue, C.; Cheng, J.; Wang, K.; Yang, Y.; Cui, D.; Parak, W.J. Investigation of the Viability of Cells upon Co-Exposure to Gold and Iron Oxide Nanoparticles. Bioconjug. Chem. 2018, 29, 2120–2125. [Google Scholar] [CrossRef] [PubMed]
- Prodan, A.M.; Iconaru, S.L.; Ciobanu, C.S.; Chifiriuc, M.C.; Stoicea, M.; Predoi, D. Iron oxide magnetic nanoparticles: Characterization and toxicity evaluation by in vitro and in vivo assays. J. Nanomater. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Zafar, H.; Zia, M.; Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [Green Version]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; De Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron oxide nanoparticles for biomedical applications: A perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vakili-Ghartavol, R.; Momtazi-Borojeni, A.A.; Vakili-Ghartavol, Z.; Aiyelabegan, H.T.; Jaafari, M.R.; Rezayat, S.M.; Arbabi Bidgoli, S. Toxicity assessment of superparamagnetic iron oxide nanoparticles in different tissues. Artif. Cells, Nanomed. Biotechnol. 2020, 48, 443–451. [Google Scholar] [CrossRef]
- Rodríguez-Galván, A.; Rivera, M.; García-López, P.; Medina, L.A.; Basiuk, V.A. Gadolinium-containing carbon nanomaterials for magnetic resonance imaging: Trends and challenges. J. Cell. Mol. Med. 2020, 24, 3779–3794. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Sun, Y.; Ma, L.; Liu, G.; Wang, Z. The Renal Clearable Magnetic Resonance Imaging Contrast Agents: State of the Art and Recent Advances. Molecules 2020, 25, 5072. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Twan, L. Iron Oxide Nanoparticles: Diagnostic, Therapeutic and Theranostic Applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Jin, R.; Lin, B.; Li, D.; Ai, H. Superparamagnetic iron oxide nanoparticles for MR imaging and therapy: Design considerations and clinical applications. Curr. Opin. Pharmacol. 2014, 18, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Wu, J.; Li, M.; Wang, P. A Novel Magnetic Nanoparticle for Early Detection of Amyloid Plaques in Alzheimer’s Disease. Arch. Med. Res. 2018, 49, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Chan, P.S.; Fan, S.; Kwan, S.M.; Yeung, K.L.; Wáng, Y.X.J.; Chow, A.H.L.; Wu, E.X.; Baum, L. Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer’s disease mice using magnetic resonance imaging (MRI). Biomaterials 2015, 44, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Niemirowicz, K.; Markiewicz, K.H.; Wilczewska, A.Z.; Car, H. Magnetic nanoparticles as new diagnostic tools in medicine. Adv. Med. Sci. 2012, 57, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Farzin, A.; Etesami, S.A.; Quint, J.; Memic, A.; Tamayol, A. Magnetic Nanoparticles in Cancer Therapy and Diagnosis. Adv. Healthc. Mater. 2020, 9, 1901058. [Google Scholar] [CrossRef] [PubMed]
- Šimečková, P.; Hubatka, F.; Kotouček, J.; Turánek Knötigová, P.; Mašek, J.; Slavík, J.; Kováč, O.; Neča, J.; Kulich, P.; Hrebík, D.; et al. Gadolinium labelled nanoliposomes as the platform for MRI theranostics: In vitro safety study in liver cells and macrophages. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- de Toledo, L.D.A.S.; Rosseto, H.C.; Bruschi, M.L. Iron oxide magnetic nanoparticles as antimicrobials for therapeutics. Pharm. Dev. Technol. 2018, 23, 316–323. [Google Scholar] [CrossRef]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005, 100, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, 167–181. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wei, J.; Aifantis, K.E.; Fan, Y.; Feng, Q.; Cui, F.Z.; Watari, F. Current investigations into magnetic nanoparticles for biomedical applications. J. Biomed. Mater. Res.-Part A 2016, 104, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Hola, K.; Markova, Z.; Zoppellaro, G.; Tucek, J.; Zboril, R. Tailored functionalization of iron oxide nanoparticles for MRI, drug delivery, magnetic separation and immobilization of biosubstances. Biotechnol. Adv. 2015, 33, 1162–1176. [Google Scholar] [CrossRef]
- Bashir, M.R.; Bhatti, L.; Marin, D.; Nelson, R.C. Emerging applications for ferumoxytol as a contrast agent in MRI. J. Magn. Reson. Imaging 2015, 41, 884–898. [Google Scholar] [CrossRef]
- Nardecchia, S.; Sánchez-Moreno, P.; de Vicente, J.; Marchal, J.A.; Boulaiz, H. Clinical trials of thermosensitive nanomaterials: An overview. Nanomaterials 2019, 9, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, D.; Wang, Q.; Zhu, T.; Wang, H.; Liu, B.; Wang, Y.; Liu, Z.; Liu, X.; Fan, D.; Wang, X. Recent Advances of Magnetic Nanomaterials in Bone Tissue Repair. Front. Chem. 2020, 8, 745. [Google Scholar] [CrossRef]
- Li, S.; Wei, C.; Lv, Y. Preparation and Application of Magnetic Responsive Materials in Bone Tissue Engineering. Curr. Stem Cell Res. Ther. 2020, 15, 428–440. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.; Zhang, M. Magnetic Nanoparticles in MR Imaging and Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.A.; Semelka, R.C. Production of net magnetization. In MRI: Basic Principles and Applications, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 1–11. ISBN 0471433101. [Google Scholar]
- Cheng, Y.N.; Haacke, E.M. Fundamental Properties of Magnetization. Curr. Protoc. Magn. Reson. Imaging 2001, 1, B1–B3. [Google Scholar] [CrossRef] [Green Version]
- What is MRI and What Can It Do? Drug Ther. Bull. 2011, 49, 141–144. [CrossRef]
- Bley, T.A.; Wieben, O.; François, C.J.; Brittain, J.H.; Reeder, S.B. Fat and water magnetic resonance imaging. J. Magn. Reson. Imaging 2010, 31, 4–18. [Google Scholar] [CrossRef]
- Bansal, R.; Hao, X.; Liu, F.; Xu, D.; Liu, J.; Peterson, B.S. The effects of changing water content, relaxation times, and tissue contrast on tissue segmentation and measures of cortical anatomy in MR images. Magn. Reson. Imaging 2013, 31, 1709–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyfer, P.; Pagenstecher, A.; Mandic, R.; Klose, K.J.; Heverhagen, J.T. Cancer and inflammation: Differentiation by USPIO-enhanced MR imaging. J. Magn. Reson. Imaging 2014, 39, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Strijkers, G.J.; Kluza, E.; Van Tilborg, G.A.F.; Van Der Schaft, D.W.J.; Griffioen, A.W.; Mulder, W.J.M.; Nicolay, K. Paramagnetic and fluorescent liposomes for target-specific imaging and therapy of tumor angiogenesis. Angiogenesis 2010, 13, 161–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima-Tenório, M.K.; Gómez Pineda, E.A.; Ahmad, N.M.; Fessi, H.; Elaissari, A. Magnetic nanoparticles: In vivo cancer diagnosis and therapy. Int. J. Pharm. 2015, 493, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Magnetic Resonance Imaging (MRI). Available online: https://www.nibib.nih.gov/science-education/science-topics/magnetic-resonance-imaging-mri (accessed on 2 May 2021).
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar]
- Fonseca, A.C.; Merwick, Á.; Dennis, M.; Ferrari, J.; Ferro, J.M.; Kelly, P.; Lal, A.; Ois, A.; Olivot, J.M.; Purroy, F. European Stroke Organisation (ESO) guidelines on management of transient ischaemic attack. Eur. Stroke J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Lim, K.S. MRI of gallbladder cancer. Diagn. Interv. Radiol. 2013, 19, 312–319. [Google Scholar] [CrossRef]
- Swayampakula, A.K.; Dillis, C.; Abraham, J. Role of MRI in screening, diagnosis and management of breast cancer. Expert Rev. Anticancer Ther. 2008, 8, 811–817. [Google Scholar] [CrossRef]
- Ayrignac, X.; Rigau, V.; Lhermitte, B.; Vincent, T.; de Champfleur, N.M.; Carra-Dalliere, C.; Charif, M.; Collongues, N.; de Seze, J.; Hebbadj, S.; et al. Pathologic and MRI analysis in acute atypical inflammatory demyelinating lesions. J. Neurol. 2019, 266, 1743–1755. [Google Scholar] [CrossRef]
- Branson, H.M. Normal Myelination. A Practical Pictorial Review. Neuroimaging Clin. N. Am. 2013, 23, 183–195. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Fox, N.C.; Jack, C.R.; Scheltens, P.; Thompson, P.M. The clinical use of structural MRI in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Femminella, G.D.; Thayanandan, T.; Calsolaro, V.; Komici, K.; Rengo, G.; Corbi, G.; Ferrara, N. Imaging and molecular mechanisms of Alzheimer’s disease: A review. Int. J. Mol. Sci. 2018, 19, 3702. [Google Scholar] [CrossRef] [Green Version]
- Chandra, A.; Dervenoulas, G.; Politis, M. Magnetic resonance imaging in Alzheimer’s disease and mild cognitive impairment. J. Neurol. 2019, 266, 1293–1302. [Google Scholar] [CrossRef] [Green Version]
- Toth, G.B.; Varallyay, C.G.; Horvath, A.; Bashir, M.R.; Choyke, P.L.; Daldrup-Link, H.E.; Dosa, E.; Finn, J.P.; Gahramanov, S.; Harisinghani, M.; et al. Current and Potential Imaging Applications of Ferumoxytol for Magnetic Resonance Imaging. Kidney Int. 2018, 92, 47–66. [Google Scholar] [CrossRef]
- Gaydos, S.S.; Varga-Szemes, A.; Judd, R.N.; Suranyi, P.; Gregg, D. Imaging in adult congenital heart disease. J. Thorac. Imaging 2017, 32, 205–216. [Google Scholar] [CrossRef]
- Qureshi, M.Y.; Sommer, R.J.; Cabalka, A.K. Tricuspid Valve Imaging and Intervention in Pediatric and Adult Patients with Congenital Heart Disease. JACC Cardiovasc. Imaging 2019, 12, 637–651. [Google Scholar] [CrossRef]
- Siripornpitak, S.; Goo, H.W. CT and MRI for Repaired Complex Adult Congenital Heart Diseases. Korean J. Radiol. 2021, 22, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Schatka, I.; Bengel, F.M. Advanced imaging of cardiac sarcoidosis. J. Nucl. Med. 2014, 55, 99–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadamura, E.; Yamamuro, M.; Kubo, S.; Kanao, S.; Saga, T.; Harada, M.; Ohba, M.; Hosokawa, R.; Kimura, T.; Kita, T.; et al. Effectiveness of delayed enhanced MRI for identification of cardiac sarcoidosis: Comparison with radionuclide imaging. Am. J. Roentgenol. 2005, 185, 110–115. [Google Scholar] [CrossRef]
- Dweck, M.R.; Puntman, V.; Vesey, A.T.; Fayad, Z.A.; Nagel, E. MR Imaging of Coronary Arteries and Plaques. JACC Cardiovasc. Imaging 2016, 9, 306–316. [Google Scholar] [CrossRef]
- Riola-Parada, C.; García-Cañamaque, L.; Pérez-Dueñas, V.; Garcerant-Tafur, M.; Carreras-Delgado, J.L. PET/RM simultánea vs. PET/TC en oncología. Una revisión sistemática. Rev. Esp. Med. Nucl. Imagen Mol. 2016, 35, 306–312. [Google Scholar] [CrossRef]
- Khiewvan, B.; Torigian, D.A.; Emamzadehfard, S.; Paydary, K.; Salavati, A.; Houshmand, S.; Werner, T.J.; Alavi, A. An update on the role of PET/CT and PET/MRI in ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Gauden, A.J.; Phal, P.M.; Drummond, K.J. MRI safety; Nephrogenic systemic fibrosis and other risks. J. Clin. Neurosci. 2010, 17, 1097–1104. [Google Scholar] [CrossRef]
- Fraum, T.J.; Ludwig, D.R.; Bashir, M.R.; Fowler, K.J. Gadolinium-based contrast agents: A comprehensive risk assessment. J. Magn. Reson. Imaging 2017, 46, 338–353. [Google Scholar] [CrossRef]
- Gallo, J.; Long, N.J.; Aboagye, E.O. Magnetic nanoparticles as contrast agents in the diagnosis and treatment of cancer. Chem. Soc. Rev. 2013, 42, 7816–7833. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.W.; Huh, Y.M.; Choi, J.S.; Lee, J.H.; Song, H.T.; Kim, S.; Yoon, S.; Kim, K.S.; Shin, J.S.; Suh, J.S.; et al. Nanoscale Size Effect of Magnetic Nanocrystals and Their Utilization for Cancer Diagnosis via Magnetic Resonance Imaging. J. Am. Chem. Soc. 2005, 127, 5732–5733. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Baek, M.J.; Choi, E.S.; Woo, S.; Kim, J.H.; Kim, T.J.; Jung, J.C.; Chae, S.; Chang, Y.; Lee, G.H. Paramagnetic Ultrasmall Gadolinium Oxide Nanoparticles as Advanced T1 MRI Contrast Agent: Account for Large Longitudinal Relaxivity, Optimal Particle Diameter, and In Vivo T1 MR Images. ACS Nano 2009, 3, 3663–3669. [Google Scholar] [CrossRef]
- Sánchez-Cabezas, S.; Montes-Robles, R.; Gallo, J.; Sancenón, F.; Martínez-Máñez, R. Combining magnetic hyperthermia and dual T1/T2 MR imaging using highly versatile iron oxide nanoparticles. Dalt. Trans. 2019, 48, 3883–3892. [Google Scholar] [CrossRef]
- Huang, X.; Yuan, Y.; Ruan, W.; Liu, L.; Liu, M.; Chen, S.; Zhou, X. pH-responsive theranostic nanocomposites as synergistically enhancing positive and negative magnetic resonance imaging contrast agents. J. Nanobiotechnol. 2018, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Zhuang, Y.; Sun, Y.; Dai, A.; Shi Xiangyang, X.; Wu, D.; Li, F.; Hu, H.; Yang, S. Targeted dual-contrast T1- and T2-weighted magnetic resonance imaging of tumors using multifunctional gadolinium-labeled superparamagnetic iron oxide nanoparticles. Biomaterials 2011, 32, 4584–4593. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Park, J.C.; Nah, H.; Woo, S.; Oh, J.; Kim, K.M.; Cheon, G.J.; Chang, Y.; Yoo, J.; Cheon, J. A hybrid nanoparticle probe for dual-modality positron emission tomography and magnetic resonance imaging. Angew. Chemie-Int. Ed. 2008, 47, 6259–6262. [Google Scholar] [CrossRef] [PubMed]
- Chircov, C.; Grumezescu, A.M.; Holban, A.M. Magnetic particles for advanced molecular diagnosis. Materials 2019, 12, 2158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnani, M.; Galluzzi, L.; Bruce, I.J. The use of magnetic nanoparticles in the development of new molecular detection systems. J. Nanosci. Nanotechnol. 2006, 6, 2302–2311. [Google Scholar] [CrossRef]
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Sirdah, M.M.; Reading, N.S. Genetic predisposition in type 2 diabetes: A promising approach toward a personalized management of diabetes. Clin. Genet. 2020, 98, 525–547. [Google Scholar] [CrossRef]
- Andrews, S.J.; McFall, G.P.; Booth, A.; Dixon, R.A.; Anstey, K.J. Association of Alzheimer’s disease genetic risk loci with cognitive performance and decline: A systematic review. J. Alzheimer’s Dis. 2019, 69, 1109–1136. [Google Scholar] [CrossRef] [Green Version]
- Hersi, M.; Irvine, B.; Gupta, P.; Gomes, J.; Birkett, N.; Krewski, D. Risk factors associated with the onset and progression of Alzheimer’s disease: A systematic review of the evidence. Neurotoxicology 2017, 61, 143–187. [Google Scholar] [CrossRef] [PubMed]
- Swarbrick, S.; Wragg, N.; Ghosh, S.; Stolzing, A. Systematic Review of miRNA as Biomarkers in Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 6156–6167. [Google Scholar] [CrossRef] [Green Version]
- Ashavaid, T.F.; Kondkar, A.A.; Dherai, A.J.; Raghavan, R.; Udani, S.V.; Udwadia, Z.F.; Desai, D. Application of multiplex ARMS and SSCP/HD analysis in molecular diagnosis of cystic fibrosis in Indian patients. Mol. Diagnosis 2005, 9, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Lakeman, I.M.M.; Schmidt, M.K.; van Asperen, C.J.; Devilee, P. Breast Cancer Susceptibility—Towards Individualised Risk Prediction. Curr. Genet. Med. Rep. 2019, 7, 124–135. [Google Scholar] [CrossRef] [Green Version]
- Amaar, Y.G.; Reeves, M.E. Research paper RASSF1C regulates miR-33a and EMT marker gene expression in lung cancer cells. Oncotarget 2019, 10, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Berenstein, R. Class III receptor tyrosine kinases in acute Leukemia-biological functions and modern laboratory analysis. Biomark. Insights 2015, 10, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, L.N.; Ismaila, N.; McShane, L.M.; Andre, F.; Collyar, D.E.; Gonzalez-Angulo, A.M.; Hammond, E.H.; Kuderer, N.M.; Liu, M.C.; Mennel, R.G.; et al. Use of biomarkers to guide decisions on adjuvant systemic therapy forwomenwith early-stage invasive breast cancer: American Society of clinical Oncology clinical practice guideline. J. Clin. Oncol. 2016, 34, 1134–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances since the 2004 Classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [Green Version]
- Papaemmanuil, E.; Ph, D.; Gerstung, M.; Ph, D.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Sc, B.; Potter, N.E.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef]
- Xu, C.; Akakuru, O.U.; Zheng, J.; Wu, A. Applications of iron oxide-based magnetic nanoparticles in the diagnosis and treatment of bacterial infections. Front. Bioeng. Biotechnol. 2019, 7, 1–15. [Google Scholar] [CrossRef]
- Wang, C.; Liu, M.; Wang, Z.; Li, S.; Deng, Y.; He, N. Point-of-care diagnostics for infectious diseases: From methods to devices. Nano Today 2021, 37, 101092. [Google Scholar] [CrossRef]
- Ngo, H.T.; Wang, H.N.; Fales, A.M.; Vo-Dinh, T. Plasmonic SERS biosensing nanochips for DNA detection. Anal. Bioanal. Chem. 2016, 408, 1773–1781. [Google Scholar] [CrossRef]
- Keshavarz, M.; Behpour, M.; Rafiee-Pour, H.A. Recent trends in electrochemical microRNA biosensors for early detection of cancer. RSC Adv. 2015, 5, 35651–35660. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, T.; Iqbal, M.Z.; Yang, F.; Hampp, N.; Wu, A.; Luo, L. Applications of magnetic materials separation in biological nanomedicine. Electrophoresis 2019, 40, 2011–2028. [Google Scholar] [CrossRef]
- Li, B.; Mou, X.; Chen, Z.; Chen, H.; Deng, Y.; Li, S.; Su, E.; He, L.; He, N. The development of a rapid high-quality universal nucleic acid extraction kit based on magnetic separation. Sci. China Chem. 2017, 60, 1602–1608. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Rutnakornpituk, B.; Theppaleak, T.; Rutnakornpituk, M.; Vilaivan, T. Recyclable magnetite nanoparticle coated with cationic polymers for adsorption of DNA. J. Biomater. Sci. Polym. Ed. 2016, 27, 1200–1210. [Google Scholar] [CrossRef]
- Kang, K.; Choi, J.; Nam, J.H.; Lee, S.C.; Kim, K.J.; Lee, S.W.; Chang, J.H. Preparation and characterization of chemically functionalized silica-coated magnetic nanoparticles as a DNA separator. J. Phys. Chem. B 2009, 113, 536–543. [Google Scholar] [CrossRef]
- Tanaka, T.; Sakai, R.; Kobayashi, R.; Hatakeyama, K.; Matsunaga, T. Contributions of phosphate to DNA adsorption/desorption behaviors on aminosilane-modified magnetic nanoparticles. Langmuir 2009, 25, 2956–2961. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Gu, H. Adsorption and desorption behaviors of DNA with magnetic mesoporous silica nanoparticles. Langmuir 2011, 27, 6099–6106. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, A.; Shibata, K.; Mogi, T.; Hosokawa, M.; Hatakeyama, K.; Gomyo, H.; Taguchi, T.; Wake, H.; Tanaami, T.; Matsunaga, T.; et al. Efficient DNA release from PAMAM dendrimer-modified superparamagnetic nanoparticles for DNA recovery. Polym. J. 2012, 44, 672–677. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.P. Magnetic nanoparticles in nanomedicine: A review of recent advances. Nanotechnology 2019, 30. [Google Scholar] [CrossRef] [Green Version]
- Labrou, N.E. Protein purification: An overview. Methods Mol. Biol. 2014, 1129, 3–10. [Google Scholar] [CrossRef]
- Safarik, I.; Safarikova, M. Magnetic techniques for the isolation and purification of proteins and peptides. Biomagn. Res. Technol. 2004, 2, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Franzreb, M.; Siemann-Herzberg, M.; Hobley, T.J.; Thomas, O.R.T. Protein purification using magnetic adsorbent particles. Appl. Microbiol. Biotechnol. 2006, 70, 505–516. [Google Scholar] [CrossRef]
- Yuan, P.; Ding, X.; Yang, Y.Y.; Xu, Q.H. Metal Nanoparticles for Diagnosis and Therapy of Bacterial Infection. Adv. Healthc. Mater. 2018, 7, 1701392. [Google Scholar] [CrossRef] [PubMed]
- Brandão, D.; Liébana, S.; Pividori, M.I. Multiplexed detection of foodborne pathogens based on magnetic particles. N. Biotechnol. 2015, 32, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, C.; Shi, L.; Shao, L.; Fu, P.; Wang, K.; Xiao, R.; Wang, S.; Gu, B. Rapid identification and antibiotic susceptibility test of pathogens in blood based on magnetic separation and surface-enhanced Raman scattering. Microchim. Acta 2019, 186. [Google Scholar] [CrossRef] [PubMed]
- Husakova, M.; Dziedzinska, R.; Slana, I. Magnetic Separation Methods for the Detection of Mycobacterium avium subsp. Paratuberculosis in Various Types of Matrices: A Review. Biomed Res. Int. 2017, 2017, 5869854. [Google Scholar] [CrossRef] [Green Version]
- Martinkova, P.; Brtnicky, M.; Kynicky, J.; Pohanka, M. Iron Oxide Nanoparticles: Innovative Tool in Cancer Diagnosis and Therapy. Adv. Healthc. Mater. 2018, 7, 1700932. [Google Scholar] [CrossRef]
- Talluri, S.; Malla, R.R. Superparamagnetic Iron Oxide Nanoparticles (SPIONs) for Diagnosis and Treatment of Breast, Ovarian and Cervical Cancers. Curr. Drug Metab. 2019, 20, 942–945. [Google Scholar] [CrossRef]
- Sosa-Acosta, J.R.; Iriarte-Mesa, C.; Ortega, G.A.; Díaz-García, A.M. DNA–Iron Oxide Nanoparticles Conjugates: Functional Magnetic Nanoplatforms in Biomedical Applications. Top. Curr. Chem. 2020, 378, 1–29. [Google Scholar] [CrossRef]
- Justin, C.; Philip, S.A.; Samrot, A.V. Synthesis and characterization of superparamagnetic iron-oxide nanoparticles (SPIONs) and utilization of SPIONs in X-ray imaging. Appl. Nanosci. 2017, 7, 463–475. [Google Scholar] [CrossRef]
- Khalid, M.; Ciracì, C. Numerical analysis of nonlocal optical response of metallic nanoshells. Photonics 2019, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.S.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, size-and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8, 3284–3307. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sahithya, C.S.; Selvarani, A.J.; Purayil, S.K.; Ponnaiah, P. A review on synthesis, characterization and potential biological applications of superparamagnetic iron oxide nanoparticles. Curr. Res. Green Sustain. Chem. 2021, 4, 100042. [Google Scholar] [CrossRef]
- Popescu, R.C.; Andronescu, E.; Vasile, B.S. Recent advances in magnetite nanoparticle functionalization for nanomedicine. Nanomaterials 2019, 9, 1791. [Google Scholar] [CrossRef] [Green Version]
- Yadav, V.K.; Ali, D.; Khan, S.H.; Gnanamoorthy, G.; Choudhary, N.; Yadav, K.K.; Thai, V.N.; Hussain, S.A.; Manhrdas, S. Synthesis and characterization of amorphous iron oxide nanoparticles by the sonochemical method and their application for the remediation of heavy metals from wastewater. Nanomaterials 2020, 10, 1551. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.; Khan, S.B.; Rehman, I.U.; Khan, M.A.; Khan, M.I. A Comprehensive Review of Magnetic Nanomaterials Modern Day Theranostics. Front. Mater. 2019, 6, 179. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef] [Green Version]
- Bensebaa, F. Wet Production Methods; Elsevier: Amsterdam, The Netherlands, 2013; Volume 19, ISBN 9780123695505. [Google Scholar]
- Umer, A.; Naveed, S.; Ramzan, N.; Rafique, M.S. Selection of a suitable method for the synthesis of copper nanoparticles. Nano 2012, 7, 1230005. [Google Scholar] [CrossRef]
- Bomatí-Miguel, O.; Tartaj, P.; Morales, M.P.; Bonville, P.; Golla-Schindler, U.; Zhao, X.Q.; Veintemillas-Verdaguer, S. Core-shell iron-iron oxide nanoparticles synthesized by laser-induced pyrolysis. Small 2006, 2, 1476–1483. [Google Scholar] [CrossRef] [Green Version]
- Gavrilović, T.V.; Jovanović, D.J.; Dramićanin, M.D. Synthesis of multifunctional inorganic materials: From micrometer to nanometer dimensions. Nanomater. Green Energy 2018, 55–81. [Google Scholar] [CrossRef]
- Eslamian, M.; Ahmed, M.; Ashgriz, N. Modelling of nanoparticle formation during spray pyrolysis. Nanotechnology 2006, 17, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- Arbain, R.; Othman, M.; Palaniandy, S. Preparation of iron oxide nanoparticles by mechanical milling. Miner. Eng. 2011, 24, 1–9. [Google Scholar] [CrossRef]
- Majidi, S.; Sehrig, F.Z.; Farkhani, S.M.; Goloujeh, M.S.; Akbarzadeh, A. Current methods for synthesis of magnetic nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 722–734. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, C.; Zhang, Z.; Wu, W.; Wang, X.; Yu, Z. Synthesis, functionalization, and nanomedical applications of functional magnetic nanoparticles. Chin. Chem. Lett. 2018, 29, 1601–1608. [Google Scholar] [CrossRef]
- Okoli, C.; Sanchez-Dominguez, M.; Boutonnet, M.; Järås, S.; Civera, C.; Solans, C.; Kuttuva, G.R. Comparison and functionalization study of microemulsion-prepared magnetic iron oxide nanoparticles. Langmuir 2012, 28, 8479–8485. [Google Scholar] [CrossRef] [PubMed]
- Bumajdad, A.; Ali, S.; Mathew, A. Characterization of iron hydroxide/oxide nanoparticles prepared in microemulsions stabilized with cationic/non-ionic surfactant mixtures. J. Colloid Interface Sci. 2011, 355, 282–292. [Google Scholar] [CrossRef]
- Cabrera, L.; Gutierrez, S.; Menendez, N.; Morales, M.P.; Herrasti, P. Magnetite nanoparticles: Electrochemical synthesis and characterization. Electrochim. Acta 2008, 53, 3436–3441. [Google Scholar] [CrossRef]
- Eid, K.; Wang, H.; Wang, L. Nanoarchitectonic Metals; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780323378307. [Google Scholar]
- Narayanan, K.B.; Sakthivel, N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interface Sci. 2010, 156, 1. [Google Scholar] [CrossRef]
- Asmathunisha, N.; Kathiresan, K. A review on biosynthesis of nanoparticles by marine organisms. Colloids Surfaces B Biointerfaces 2013, 103, 283–287. [Google Scholar] [CrossRef]
- Ash, A.; Revati, K.; Pandey, B.D. Microbial synthesis of iron-based nanomaterials—A review. Bull. Mater. Sci. 2011, 34, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Kurapov, Y.A.; Vazhnichaya, E.M.; Litvin, S.E.; Romanenko, S.M.; Didikin, G.G.; Devyatkina, T.A.; Mokliak, Y.V.; Oranskaya, E.I. Physical synthesis of iron oxide nanoparticles and their biological activity in vivo. SN Appl. Sci. 2019, 1, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S. Recent advances in inorganic nanomaterials synthesis using sonochemistry: A comprehensive review on iron oxide, gold and iron oxide coated gold nanoparticles. Molecules 2021, 26, 2453. [Google Scholar] [CrossRef]
- Ajinkya, N.; Yu, X.; Kaithal, P.; Luo, H.; Somani, P.; Ramakrishna, S. Magnetic iron oxide nanoparticle (IONP) synthesis to applications: Present and future. Materials 2020, 13, 4644. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, D.Y.; Zhang, Y.Z.; Kang, Z. Preparation of magnesium ferrite nanoparticles by ultrasonic wave-assisted aqueous solution ball milling. Ultrason. Sonochem. 2013, 20, 1337–1340. [Google Scholar] [CrossRef]
- Duguet, E.; Vasseur, S.; Mornet, S.; Devoisselle, J.-M. Magnetic nanoparticles and their applications in medicine. Nanomedicine 2006, 1, 157–168. [Google Scholar] [CrossRef]
- European Medicines Agency: EMA/437901/2015—Rienso, Withdrawal of the Marketing Authorisation in the European Union; European Medicines Agency: London, UK, 2015.
- European Medicines Agency: EMA/102191/2015—EPAR Summary for the Public Rienso. Available online: https://www.ema.europa.eu/en/documents/overview/rienso-epar-summary-public_en.pdf (accessed on 11 July 2021).
- Leiner, T.; Ho, K.Y.J.A.M.; Ho, V.B.; Bongartz, G.; Mali, W.P.T.M.; Engelshoven, J.M.A. van Multicenter phase-II trial of safety and efficacy of NC100150 for steady-state contrast-enhanced peripheral magnetic resonance angiography. Eur. Radiol. 2003, 13, 1620–1627. [Google Scholar] [CrossRef]
- Weishaupt, D.; Rühm, S.G.; Binkert, C.A.; Schmidt, M.; Patak, M.A.; Steybe, F.; McGill, S.; Debatin, J.F. Equilibrium-phase MR angiography of the aortoiliac and renal arteries using a blood pool contrast agent. Am. J. Roentgenol. 2000, 175, 189–195. [Google Scholar] [CrossRef]
- Schneider, M.G.M.; Lassalle, V.L. Magnetic iron oxide nanoparticles as novel and efficient tools for atherosclerosis diagnosis. Biomed. Pharmacother. 2017, 93, 1098–1115. [Google Scholar] [CrossRef]
- Taupitz, M.; Wagner, S.; Schnorr, J.; Kravec, I.; Pilgrimm, H.; Bergmann-Fritsch, H.; Hamm, B. Phase I clinical evaluation of citrate-coated monocrystalline very small superparamagnetic iron oxide particles as a new contrast medium for magnetic resonance imaging. Investig. Radiol. 2004, 39, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Wagner, S.; Schnorr, J.; Schellenberger, E.; Kivelitz, D.; Krug, L.; Dewey, M.; Laule, M.; Hamm, B.; Taupitz, M. Coronary MR angiography using citrate-coated very small superparamagnetic iron oxide particles as blood-pool contrast agent: Initial experience in humans. J. Magn. Reson. Imaging 2011, 34, 816–823. [Google Scholar] [CrossRef]
- Schnorr, J.; Wagner, S.; Abramjuk, C.; Wojner, I.; Schink, T.; Kroencke, T.J.; Schellenberger, E.; Hamm, B.; Pilgrimm, H.; Taupitz, M. Comparison of the iron oxide-based blood-pool contrast medium VSOP-C184 with gadopentetate dimeglumine for first-pass magnetic resonance angiography of the aorta and renal arteries in pigs. Investig. Radiol. 2004, 39, 546–553. [Google Scholar] [CrossRef]
- Teshome, M.; Wei, C.; Hunt, K.K.; Thompson, A.; Rodriguez, K.; Mittendorf, E.A. Use of a Magnetic Tracer for Sentinel Lymph Node Detection in Early-Stage Breast Cancer Patients: A Meta-analysis. Ann. Surg. Oncol. 2016, 23, 1508–1514. [Google Scholar] [CrossRef]
- Wáng, Y.X.J.; Idée, J.M. A comprehensive literatures update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging. Quant. Imaging Med. Surg. 2017, 7, 88–122. [Google Scholar] [CrossRef] [Green Version]
- Norouzi, M.; Derakhshanfar, S.; Kazemian, M.R.; França, R. Superparamagnetic Iron Oxide Nanoparticles in Cancer Theranostics. J. Appl. Mater. Sci. Eng. Res. 2018, 2, 1–6. [Google Scholar]
- Oude Engberink, R.D.; Van Der Pol, S.M.A.; Walczak, P.; Van Der Toorn, A.; Viergever, M.A.; Dijkstra, C.D.; Bulte, J.W.M.; De Vries, H.E.; Blezer, E.L.A. Magnetic resonance imaging of monocytes labeled with ultrasmall superparamagnetic particles of iron oxide using magnetoelectroporation in an animal model of multiple sclerosis. Mol. Imaging 2010, 9, 268–277. [Google Scholar] [CrossRef]
- Thakor, A.S.; Jokerst, J.V.; Ghanouni, P.; Campbell, J.L.; Mittra, E.; Gambhir, S.S. Clinically Approved Nanoparticle Imaging Agents. J. Nucl. Med. 2016, 57, 1833–1837. [Google Scholar] [CrossRef] [Green Version]
- Modo, M.; Kolosnjaj-Tabi, J.; Nicholls, F.; Ling, W.; Wilhelm, C.; Debarge, O.; Gazeau, F.; Clement, O. Considerations for the clinical use of contrast agents for cellular MRI in regenerative medicine. Contrast Media Mol. Imaging 2013, 8, 439–455. [Google Scholar] [CrossRef] [PubMed]
- AMAG Pharmaceuticals and Takeda Announce Mutual Termination of Agreement to License, Develop and Commercialize Ferumoxytol in Ex-U.S. Territories, Including Europe. Available online: https://www.takeda.com/newsroom/newsreleases/2015/amag-pharmaceuticals-and-takeda-announce-mutual-termination-of-agreement-to-license-develop-and-commercialize-ferumoxytol-in-ex-u.s.-territories-including-europe/ (accessed on 11 July 2021).
- Neuwelt, E.A.; Hamilton, B.E.; Varallyay, C.G.; Rooney, W.R.; Edelman, R.D.; Jacobs, P.M.; Watnick, S.G. Ultrasmall superparamagnetic iron oxides (USPIOs): A future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF)? Kidney Int. 2009, 75, 465–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, K. Ferumoxides—Molecular Imaging and Contrast Agent Database (MICAD)—NCBI Bookshelf. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/books/NBK23037/#!po=58.3333 (accessed on 14 July 2021).
- Karussis, D.; Karageorgiou, C.; Vaknin-Dembinsky, A.; Gowda-Kurkalli, B.; Gomori, J.M.; Kassis, I.; Bulte, J.W.M.; Petrou, P.; Ben-Hur, T.; Abramsky, O.; et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch. Neurol. 2010, 67, 1187–1194. [Google Scholar] [CrossRef]
- Leung, K. Ferumoxsil—Molecular Imaging and Contrast Agent Database (MICAD)—NCBI Bookshelf. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/books/NBK22994/ (accessed on 22 July 2021).
- Polakova, K.; Mocikova, I.; Purova, D.; Tucek, P.; Novak, P.; Novotna, K.; Niko, I.; Bielik, R.; Zboril, R.; Herman, M. Magnetic resonance cholangiopancreatography (MRCP) using new negative per-oral contrast agent based on superparamagnetic iron oxide nanoparticles for extrahepatic biliary duct visualization in liver cirrhosis. Biomed. Pap. 2016, 160, 512–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- STEMCELL Technologies. Available online: www.stemcell.com (accessed on 20 August 2021).
- Chemicell. Available online: www.chemicell.com (accessed on 20 August 2021).
- Dexter Magnetic. Technologies. Available online: www.dextermag.com (accessed on 20 August 2021).
- Ocean NanoTech. Available online: www.oceannanotech.com (accessed on 20 August 2021).
- TurboBeads. Available online: www.turbobeads.com (accessed on 20 August 2021).
- SEPMAG. Available online: www.sepmag.eu (accessed on 20 August 2021).
- Merck. Available online: www.merckmillipore.com (accessed on 21 August 2021).
- Miltenyi Biotec. Available online: www.miltenyibiotec.com (accessed on 21 August 2021).
- Invitrogen. Available online: www.thermofisher.com/invitrogen (accessed on 21 August 2021).
- Cube Biotech. Available online: www.cube-biotech.com (accessed on 21 August 2021).
- European Medicines Agency: EMEA/CHMP/11527/2008—Withdrawal Assessment report For Sinerem; European Medicines Agency: London, UK, 2008.
- Leung, K. Ferumoxtran—Molecular Imaging and Contrast Agent Database (MICAD)—NCBI Bookshelf. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/books/NBK23402/ (accessed on 25 July 2021).
- Tu, C.; Louie, A.Y. Nanoformulations for molecular MRI. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 448–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, X.; Zhu, Q.; Zeng, Y.; Zeng, Q.; Chen, X.; Zhan, Y. Manganese oxide nanoparticles as mri contrast agents in tumor multimodal imaging and therapy. Int. J. Nanomed. 2019, 14, 8321–8344. [Google Scholar] [CrossRef] [Green Version]
- Unger, E.C.; Shen, D.-K.; Fritz, T.A. Status of liposomes as MR contrast agents. J. Magn. Reson. Imaging 1993, 3, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers Jessica. Physiol. Behav. 2019, 119, 957–1057. [Google Scholar] [CrossRef] [PubMed]
- Waris, A.; Din, M.; Ali, A.; Afridi, S.; Baset, A.; Khan, A.U.; Ali, M. Green fabrication of Co and Co3O4nanoparticles and their biomedical applications: A review. Open Life Sci. 2021, 16, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Mauri, M.; Collico, V.; Morelli, L.; Das, P.; García, I.; Penaranda Avila, J.; Bellini, M.; Rotem, R.; Truffi, M.; Corsi, F.; et al. MnO Nanoparticles Embedded in Functional Polymers as T1 Contrast Agents for Magnetic Resonance Imaging. ACS Appl. Nano Mater. 2020, 3, 3787–3797. [Google Scholar] [CrossRef]
- Natalin, R.A.; Prince, M.R.; Grossman, M.E.; Silvers, D.; Landman, J. Contemporary Applications and Limitations of Magnetic Resonance Imaging Contrast Materials. J. Urol. 2010, 183, 27–33. [Google Scholar] [CrossRef]
- Zeng, L.; Wu, D.; Zou, R.; Chen, T.; Zhang, J.; Wu, A. Paramagnetic and Superparamagnetic Inorganic Nanoparticles for T1-Weighted Magnetic Resonance Imaging. Curr. Med. Chem. 2017, 25, 2970–2986. [Google Scholar] [CrossRef]
- Jacques, V.; Dumas, S.; Sun, W.-C.; Troughton, J.S.; Greenfield, M.T.; Caravan, P. High relaxivity MRI contrast agents part 2: Optimization of inner and second-sphere relaxivity. Investig. Radiol. 2010, 45, 613–624. [Google Scholar] [CrossRef] [Green Version]
- Yamada, K. Cobalt: Its role in health and disease. Met. Ions Life Sci. 2013, 13, 295–320. [Google Scholar] [CrossRef]
- Srinivasan, S.Y.; Paknikar, K.M.; Bodas, D.; Gajbhiye, V. Applications of cobalt ferrite nanoparticles in biomedical nanotechnology. Nanomedicine 2018, 13, 1221–1238. [Google Scholar] [CrossRef]
- Abudayyak, M.; Altincekic Gurkaynak, T.; Özhan, G. In Vitro Toxicological Assessment of Cobalt Ferrite Nanoparticles in Several Mammalian Cell Types. Biol. Trace Elem. Res. 2017, 175, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Abudayyak, M.; Altinçekiç Guekaynak, T.; Özhan, G. In Vitro Evaluation of the Toxicity of Cobalt Ferrite Nanoparticles in Kidney Cell. Turkish J. Pharm. Sci. 2017, 14, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Zhou, Y. Pitfalls and Challenges in Nanotoxicology: A Case of Cobalt Ferrite (CoFe2O4) Nanocomposites. Chem. Res. Toxicol. 2017, 30, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, M.; Gao, Z.; Du, J.; Yang, W.; Yin, M. Effective approach towards Si-bilayer-IDA modified CoFe2O4 magnetic nanoparticles for high efficient protein separation. Colloids Surfaces B Biointerfaces 2016, 146, 468–474. [Google Scholar] [CrossRef]
- Sun, C.; Hassanisaber, H.; Yu, R.; Ma, S.; Verbridge, S.S.; Lu, C. Paramagnetic Structures within a Microfluidic Channel for Enhanced Immunomagnetic Isolation and Surface Patterning of Cells. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Bohara, R.A.; Throat, N.D.; Mulla, N.A.; Pawar, S.H. Surface-Modified Cobalt Ferrite Nanoparticles for Rapid Capture, Detection, and Removal of Pathogens: A Potential Material for Water Purification. Appl. Biochem. Biotechnol. 2017, 182, 598–608. [Google Scholar] [CrossRef]
- Chen, Z.G.; Tang, D.Y. Antigen-antibody interaction from quartz crystal microbalance immunosensors based on magnetic CoFe2O4/SiO2 composite nanoparticle-functionalized biomimetic interface. Bioprocess Biosyst. Eng. 2007, 30, 243–249. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Yang, X.; Xie, S.; Yuan, R.; Chai, Y. Metal Organic Frameworks Combining CoFe2O4 Magnetic Nanoparticles as Highly Efficient SERS Sensing Platform for Ultrasensitive Detection of N-Terminal Pro-Brain Natriuretic Peptide. ACS Appl. Mater. Interfaces 2016, 8, 7683–7690. [Google Scholar] [CrossRef]
- O’Neal, S.L.; Zheng, W. Manganese Toxicity Upon Overexposure: A Decade in Review. Curr. Environ. Health reports 2015, 2, 315–328. [Google Scholar] [CrossRef] [Green Version]
- Manganese Contrast and Teslascan. Available online: http://mriquestions.com/mn-agents-teslascan.html (accessed on 2 August 2021).
- Youk, J.H.; Lee, J.M.; Kim, C.S. MRI for detection of hepatocellular carcinoma: Comparison of mangafodipir trisodium and gadopentetate dimeglumine contrast agents. Am. J. Roentgenol. 2004, 183, 1049–1054. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency: EMA/486286/2012—Public Statement on Teslascan (Mangafodipir) Withdrawal of the Marketing Authorisation in the European Union; European Medicines Agency: London, UK, 2010.
- LumenHanceTM—U.S. Food and Drug Administration Search Results. Available online: https://search.usa.gov/search?query=LumenHanceTM&affiliate=fda1 (accessed on 12 August 2021).
- Li, J.; Wu, C.; Hou, P.; Zhang, M.; Xu, K. One-pot preparation of hydrophilic manganese oxide nanoparticles as T1 nano-contrast agent for molecular magnetic resonance imaging of renal carcinoma in vitro and in vivo. Biosens. Bioelectron. 2018, 102, 1–8. [Google Scholar] [CrossRef]
- Chen, N.; Shao, C.; Qu, Y.; Li, S.; Gu, W.; Zheng, T.; Ye, L.; Yu, C. Folic acid-conjugated MnO nanoparticles as a T1 contrast agent for Magnetic Resonance imaging of tiny brain gliomas. ACS Appl. Mater. Interfaces 2014, 6, 19850–19857. [Google Scholar] [CrossRef] [PubMed]
- Douglas, F.J.; Maclaren, D.A.; Tuna, F.; Holmes, W.M.; Berry, C.C.; Murrie, M. Formation of octapod MnO nanoparticles with enhanced magnetic properties through kinetically-controlled thermal decomposition of polynuclear manganese complexes. Nanoscale 2014, 6, 172–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, X.Y.; Li, J.Y.; Sheng, D.; Lian, H.Z. Spinel-type manganese ferrite (MnFe2O4) microspheres: A novel affinity probe for selective and fast enrichment of phosphopeptides. Talanta 2017, 166, 36–45. [Google Scholar] [CrossRef]
- Long, X.Y.; Zhang, Z.J.; Li, J.Y.; Sheng, D.; Lian, H.Z. Controllable Preparation of CuFeMnO4 Nanospheres as a Novel Multifunctional Affinity Probe for Efficient Adsorption and Selective Enrichment of Low-Abundance Peptides and Phosphopeptides. Anal. Chem. 2017, 89, 10446–10453. [Google Scholar] [CrossRef]
- Ni, D.; Bu, W.; Ehlerding, E.B.; Cai, W.; Shi, J. Engineering of inorganic nanoparticles as magnetic resonance imaging contrast agents. Physiol. Behav. 2017, 46, 7438–7468. [Google Scholar] [CrossRef]
- Kattel, K.; Park, J.Y.; Xu, W.; Kim, H.G.; Lee, E.J.; Bony, B.A.; Heo, W.C.; Jin, S.; Baeck, J.S.; Chang, Y.; et al. Paramagnetic dysprosium oxide nanoparticles and dysprosium hydroxide nanorods as T2 MRI contrast agents. Biomaterials 2012, 33, 3254–3261. [Google Scholar] [CrossRef]
- González-Mancebo, D.; Becerro, A.I.; Rojas, T.C.; García-Martín, M.L.; de la Fuente, J.M.; Ocaña, M. HoF3 and DyF3 Nanoparticles as Contrast Agents for High-Field Magnetic Resonance Imaging. Part. Part. Syst. Charact. 2017, 34, 1700116. [Google Scholar] [CrossRef]
- Atabaev, T.S.; Shin, Y.C.; Song, S.J.; Han, D.W.; Hong, N.H. Toxicity and t2-weighted magnetic resonance imaging potentials of holmium oxide nanoparticles. Nanomaterials 2017, 7, 216. [Google Scholar] [CrossRef] [Green Version]
- Kattel, K.; Park, J.Y.; Xu, W.; Kim, H.G.; Lee, E.J.; Bony, B.A.; Heo, W.C.; Lee, J.J.; Jin, S.; Baeck, J.S.; et al. A facile synthesis, in vitro and in vivo MR studies of d-glucuronic acid-coated ultrasmall Ln2O3 (Ln = Eu, Gd, Dy, Ho, and Er) nanoparticles as a new potential MRI contrast agent. ACS Appl. Mater. Interfaces 2011, 3, 3325–3334. [Google Scholar] [CrossRef]
- Yue, H.; Park, J.Y.; Chang, Y.; Lee, G.H. Ultrasmall Europium, Gadolinium, and Dysprosium Oxide Nanoparticles: Polyol Synthesis, Properties, and Biomedical Imaging Applications. Mini-Reviews Med. Chem. 2020, 20, 1767–1780. [Google Scholar] [CrossRef]
- Park, J.Y.; Chang, Y.; Lee, G.H. Multi-Modal Imaging and Cancer Therapy Using Lanthanide Oxide Nanoparticles: Current Status and Perspectives. Curr. Med. Chem. 2014, 22, 569–581. [Google Scholar] [CrossRef]
- Shirshahi, V.; Soltani, M. Solid silica nanoparticles: Applications in molecular imaging. Contrast Media Mol. Imaging 2015, 10, 1–17. [Google Scholar] [CrossRef]
- Li, X.; Zhao, W.; Liu, X.; Chen, K.; Zhu, S.; Shi, P.; Chen, Y.; Shi, J. Mesoporous manganese silicate coated silica nanoparticles as multi-stimuli-responsive T1-MRI contrast agents and drug delivery carriers. Acta Biomater. 2016, 30, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Morimoto, H.; Nakagawa, T.; Kubota, Y.; Gonda, K.; Ohuchi, N. Preparation of Gd Complex-Immobilized Silica Particles and Their Application to MRI. ISRN Nanotechnol. 2013, 2013, 908614. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Song, M.; Cui, Y.; Shi, C.; Wang, D.; Paoli, G.C.; Shi, X. A rapid method for the detection of foodborne pathogens by extraction of a trace amount of DNA from raw milk based on amino-modified silica-coated magnetic nanoparticles and polymerase chain reaction. Anal. Chim. Acta 2013, 787, 93–101. [Google Scholar] [CrossRef]
- Jia, X.; Xu, M.; Wang, Y.; Ran, D.; Yang, S.; Zhang, M. Polydopamine-based molecular imprinting on silica-modified magnetic nanoparticles for recognition and separation of bovine hemoglobin. Analyst 2013, 138, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Pham, X.H.; Hahm, E.; Kim, H.M.; Son, B.S.; Jo, A.; An, J.; Thi, T.A.T.; Nguyen, D.Q.; Jun, B.H. Silica-coated magnetic iron oxide nanoparticles grafted onto graphene oxide for protein isolation. Nanomaterials 2020, 10, 117. [Google Scholar] [CrossRef] [Green Version]
- Kyeong, S.; Jeong, C.; Kang, H.; Cho, H.J.; Park, S.J.; Yang, J.K.; Kim, S.; Kim, H.M.; Jun, B.H.; Lee, Y.S. Double-layer magnetic nanoparticle-embedded silica particles for efficient bio-separation. PLoS ONE 2015, 10, e0143727. [Google Scholar] [CrossRef]
- Liu, R.; Jing, L.; Peng, D.; Li, Y.; Tian, J.; Dai, Z. Manganese (II) chelate functionalized copper sulfide nanoparticles for efficient magnetic resonance/ photoacoustic dual-modal imaging guided photothermal therapy. Theranostics 2015, 5, 1144–1153. [Google Scholar] [CrossRef] [Green Version]
- Poudel, K.; Gautam, M.; Jin, S.G.; Choi, H.G.; Yong, C.S.; Kim, J.O. Copper sulfide: An emerging adaptable nanoplatform in cancer theranostics. Int. J. Pharm. 2019, 562, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Chen, F.; Cai, W. Synthesis and biomedical applications of copper sulfide nanoparticles: From sensors to theranostics. Small 2014, 10, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, L.; Zhao, Y.; Sun, L.; Zhang, Y. Selective binding and magnetic separation of histidine-tagged proteins using Fe3O4/Cu-apatite nanoparticles. J. Inorg. Biochem. 2016, 156, 49–54. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Zhang, Y.; Zhang, D.D.; Shu, Y.; Chen, X.W.; Wang, J.H. Magnetic Nanospheres Encapsulated by Mesoporous Copper Oxide Shell for Selective Isolation of Hemoglobin. ACS Appl. Mater. Interfaces 2016, 8, 29734–29741. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Ruijun, X.; Zhichuon, X.; Yonglong, H.; Song, G.; Shouhene, S. Synthesis, functionalization, and biomedical applications of multifunctional magnetic nanoparticles. Adv. Mater. 2010, 22, 2729–2742. [Google Scholar] [CrossRef]
- Amendola, V.; Torresan, V.; Forrer, D.; Guadagnini, A.; Badocco, D.; Pastore, P.; Casarin, M.; Selloni, A.; Coral, D.; Ceolin, M.; et al. 4d multimodal nanomedicines made of nonequilibrium au-fe alloy nanoparticles. ACS Nano 2020, 14, 12840–12853. [Google Scholar] [CrossRef]
- Hütten, A.; Sudfeld, D.; Ennen, I.; Reiss, G.; Hachmann, W.; Heinzmann, U.; Wojczykowski, K.; Jutzi, P.; Saikaly, W.; Thomas, G. New magnetic nanoparticles for biotechnology. J. Biotechnol. 2004, 112, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.E.; Van de Walle, A.; Wilhelm, C. Versatile iron cobalt nanoparticles for theranostics. Nat. Biomed. Eng. 2020, 4, 252–253. [Google Scholar] [CrossRef]
- Huynh, K.H.; Pham, X.H.; Kim, J.; Lee, S.H.; Chang, H.; Rho, W.Y.; Jun, B.H. Synthesis, properties, and biological applications of metallic alloy nanoparticles. Int. J. Mol. Sci. 2020, 21, 5174. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farinha, P.; Coelho, J.M.P.; Reis, C.P.; Gaspar, M.M. A Comprehensive Updated Review on Magnetic Nanoparticles in Diagnostics. Nanomaterials 2021, 11, 3432. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123432
Farinha P, Coelho JMP, Reis CP, Gaspar MM. A Comprehensive Updated Review on Magnetic Nanoparticles in Diagnostics. Nanomaterials. 2021; 11(12):3432. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123432
Chicago/Turabian StyleFarinha, Pedro, João M. P. Coelho, Catarina Pinto Reis, and Maria Manuela Gaspar. 2021. "A Comprehensive Updated Review on Magnetic Nanoparticles in Diagnostics" Nanomaterials 11, no. 12: 3432. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123432