Surfactant-Free Monodispersed Pd Nanoparticles Template for Core-Shell Pd@PdPt Nanoparticles as Electrocatalyst towards Methanol Oxidation Reaction (MOR)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Electrocatalyst
2.1.1. Preparation of Pd/CNTs Template Composite Precursor
2.1.2. Preparation of Pd@PdPt/CNTs
2.2. Materials Characterization
2.3. Electrochemical Measurements
3. Results and Discussions
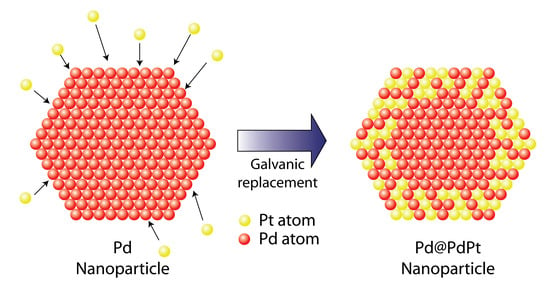
3.1. Synthesis of Pd@PdPt/CNTs Electrocatalyst
3.2. Physical Characterization of Electrocatalysts
3.3. Electrochemical Characterization of Pd@PdPt/CNTs Electrocatalyst
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ding, L.X.; Li, G.R.; Wang, Z.L.; Liu, Z.Q.; Liu, H.; Tong, Y.X. Porous Ni@ Pt Core-Shell Nanotube Array Electrocatalyst with High Activity and Stability for Methanol Oxidation. Chem. A Eur. J. 2012, 18, 8386–8391. [Google Scholar] [CrossRef]
- Zhao, X.; Yin, M.; Ma, L.; Liang, L.; Liu, C.; Liao, J.; Lu, T.; Xing, W. Recent advances in catalysts for direct methanol fuel cells. Energy Environ. Sci. 2011, 4, 2736–2753. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.; Sun, K.; Zou, S.; Fang, J. Enhancing by weakening: Electrooxidation of methanol on Pt3Co and Pt nanocubes. Angew. Chem. 2010, 122, 7000–7003. [Google Scholar] [CrossRef]
- Yang, X.; Zhen, M.; Li, G.; Liu, X.; Wang, X.; Shu, C.; Jiang, L.; Wang, C. Preparation of Pd-decorated fullerenols on carbon nanotubes with excellent electrocatalytic properties in alkaline media. J. Mater. Chem. A 2013, 1, 8105–8110. [Google Scholar] [CrossRef]
- Gong, L.; Yang, Z.; Li, K.; Xing, W.; Liu, C.; Ge, J. Recent development of methanol electrooxidation catalysts for direct methanol fuel cell. J. Energy Chem. 2018, 27, 1618–1628. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, L.; Chen, T.; Yu, H.; Zhang, L.; Xue, H.; Hu, H. Supercritical carbon-dioxide-assisted deposition of Pt nanoparticles on graphene sheets and their application as an electrocatalyst for direct methanol fuel cells. J. Phys. Chem. C 2012, 116, 21374–21381. [Google Scholar] [CrossRef]
- Yang, H.; Geng, L.; Zhang, Y.; Chang, G.; Zhang, Z.; Liu, X.; Lei, M.; He, Y. Graphene-templated synthesis of palladium nanoplates as novel electrocatalyst for direct methanol fuel cell. Appl. Surf. Sci. 2019, 466, 385–392. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y.; Li, S. Self-assembled platinum nanoparticles on sulfonic acid-grafted graphene as effective electrocatalysts for methanol oxidation in direct methanol fuel cells. Sci. Rep. 2016, 6, 21530. [Google Scholar] [CrossRef]
- Xia, Z.; Wang, S.; Li, Y.; Jiang, L.; Sun, H.; Zhu, S.; Su, D.S.; Sun, G. Vertically oriented polypyrrole nanowire arrays on Pd-plated Nafion® membrane and its application in direct methanol fuel cells. J. Mater. Chem. A 2013, 1, 491–494. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Shyam, B.; Nishijima, M.; Yang, X.; Koel, B.; Ernst, F.; Ramaker, D.; Mukerjee, S. Highly stable Pt–Au@ Ru/C catalyst nanoparticles for methanol electro-oxidation. J. Phys. Chem. C 2013, 117, 1457–1467. [Google Scholar] [CrossRef]
- Cui, X.; Wang, X.; Xu, X.; Yang, S.; Wang, Y. One-step stabilizer-free synthesis of porous bimetallic PdCu nanofinger supported on graphene for highly efficient methanol electro-oxidation. Electrochim. Acta 2018, 260, 47–54. [Google Scholar] [CrossRef]
- Zheng, F.; Luk, S.-Y.; Kwong, T.-L.; Yung, K.-F. Synthesis of hollow PtAg alloy nanospheres with excellent electrocatalytic performances towards methanol and formic acid oxidations. RSC Adv. 2016, 6, 44902–44907. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-S.; Yang, S.-Y.; Li, S.-M.; Tien, H.-W.; Hsiao, S.-T.; Liao, W.-H.; Liu, C.-H.; Chang, K.-H.; Ma, C.-C.M.; Hu, C.-C. Three-dimensionally porous graphene—Carbon nanotube composite-supported PtRu catalysts with an ultrahigh electrocatalytic activity for methanol oxidation. Electrochim. Acta 2013, 87, 261–269. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K.; Bahru, R.; Osman, S.H.; Md Ishak, N.A.I. Progress and challenges: Review for direct liquid fuel cell. Int. J. Energy Res. 2021, 45, 6644–6688. [Google Scholar] [CrossRef]
- Chen, A.; Holt-Hindle, P. Platinum-based nanostructured materials: Synthesis, properties, and applications. Chem. Rev. 2010, 110, 3767–3804. [Google Scholar] [CrossRef]
- Stephen, A.; Rees, N.; Mikheenko, I.; Macaskie, L.E. Platinum and Palladium Bio-Synthesized Nanoparticles as Sustainable Fuel Cell Catalysts. Front. Energy Res. 2019, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Anson, C.W.; Stahl, S.S. Mediated Fuel Cells: Soluble Redox Mediators and Their Applications to Electrochemical Reduction of O2 and Oxidation of H2, Alcohols, Biomass, and Complex Fuels. Chem. Rev. 2020, 120, 3749–3786. [Google Scholar] [CrossRef]
- Bu, L.; Zhang, N.; Guo, S.; Zhang, X.; Li, J.; Yao, J.; Wu, T.; Lu, G.; Ma, J.-Y.; Su, D. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 2016, 354, 1410–1414. [Google Scholar] [CrossRef]
- Du, H.; Luo, S.; Wang, K.; Tang, M.; Sriphathoorat, R.; Jin, Y.; Shen, P.K. High-quality and deeply excavated Pt3Co nanocubes as efficient catalysts for liquid fuel electrooxidation. Chem. Mater. 2017, 29, 9613–9617. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, R.; Ren, J.; Dai, Y.; Yuan, Y.; Wang, Z. PtFeCu Concave Octahedron Nanocrystals as Electrocatalysts for the Methanol Oxidation Reaction. Langmuir 2019, 35, 16752–16760. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Li, S.; Song, G.; Zha, P.; Li, C.; Wei, Q.; Lv, Y.; Chen, G. One-pot synthesis of Pt− Cu bimetallic nanocrystals with different structures and their enhanced electrocatalytic properties. Nano Res. 2018, 11, 2612–2624. [Google Scholar] [CrossRef]
- Huang, H.; Hu, X.; Zhang, J.; Su, N.; Cheng, J. Facile fabrication of platinum-cobalt alloy nanoparticles with enhanced electrocatalytic activity for a methanol oxidation reaction. Sci. Rep. 2017, 7, 45555. [Google Scholar] [CrossRef]
- Wu, H.; Li, H.; Zhai, Y.; Xu, X.; Jin, Y. Facile synthesis of free-standing Pd-based nanomembranes with enhanced catalytic performance for methanol/ethanol oxidation. Adv. Mater. 2012, 24, 1594–1597. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Y.H.; Mohamed, A.T.; Alashraf, A.; Matalqeh, M.; El-Shafei, A.; Al-Qaradawi, S.Y.; Aljaber, A.S. Highly porous PtPd nanoclusters synthesized via selective chemical etching as efficient catalyst for ethanol electro-oxidation. Appl. Surf. Sci. 2020, 508, 145222. [Google Scholar] [CrossRef]
- Chu, Y.-Y.; Wang, Z.-B.; Jiang, Z.-Z.; Gu, D.-M.; Yin, G.-P. Facile synthesis of hollow spherical sandwich PtPd/C catalyst by electrostatic self-assembly in polyol solution for methanol electrooxidation. J. Power Sour. 2012, 203, 17–25. [Google Scholar] [CrossRef]
- Jin, X.; Zhao, M.; Vora, M.; Shen, J.; Zeng, C.; Yan, W.; Thapa, P.S.; Subramaniam, B.; Chaudhari, R.V. Synergistic effects of bimetallic PtPd/TiO2 nanocatalysts in oxidation of glucose to glucaric acid: Structure dependent activity and selectivity. Ind. Eng. Chem. Res. 2016, 55, 2932–2945. [Google Scholar] [CrossRef]
- Zhou, Y.; Du, C.; Han, G.; Gao, Y.; Yin, G. Ultra-low Pt decorated PdFe alloy nanoparticles for formic acid electro-oxidation. Electrochim. Acta 2016, 217, 203–209. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Ganesan, P.; Popov, B.N. Electrocatalytic activity and stability of titania-supported platinum–palladium electrocatalysts for polymer electrolyte membrane fuel cell. ACS Catal. 2012, 2, 825–831. [Google Scholar] [CrossRef]
- Xiong, X.; Chen, W.; Wang, W.; Li, J.; Chen, S. Pt-Pd nanodendrites as oxygen reduction catalyst in polymer-electrolyte-membrane fuel cell. Int. J. Hydrog. Energy 2017, 42, 25234–25243. [Google Scholar] [CrossRef]
- Hong, J.W.; Kang, S.W.; Choi, B.-S.; Kim, D.; Lee, S.B.; Han, S.W. Controlled synthesis of Pd–Pt alloy hollow nanostructures with enhanced catalytic activities for oxygen reduction. ACS Nano 2012, 6, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-S.; Lee, Y.W.; Kang, S.W.; Hong, J.W.; Kim, J.; Park, I.; Han, S.W. Multimetallic alloy nanotubes with nanoporous framework. ACS Nano 2012, 6, 5659–5667. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, S.; Dong, S. PdM (M = Pt, Au) Bimetallic Alloy Nanowires with Enhanced Electrocatalytic Activity for Electro-oxidation of Small Molecules. Adv. Mater. 2012, 24, 2326–2331. [Google Scholar] [CrossRef]
- Chen, C.-S.; Pan, F.-M. Effects of the PdO nanoflake support on electrocatalytic activity of Pt nanoparticles toward methanol oxidation in acidic solutions. J. Power Sour. 2012, 208, 9–17. [Google Scholar] [CrossRef]
- Huang, L.; Yang, J.; Wu, M.; Shi, Z.; Lin, Z.; Kang, X.; Chen, S. PdAg@ Pd core-shell nanotubes: Superior catalytic performance towards electrochemical oxidation of formic acid and methanol. J. Power Sour. 2018, 398, 201–208. [Google Scholar] [CrossRef]
- Park, H.-Y.; Park, J.H.; Kim, P.; Yoo, S.J. Hollow PdCu2@ Pt core@ shell nanoparticles with ordered intermetallic cores as efficient and durable oxygen reduction reaction electrocatalysts. Appl. Catal. B Environ. 2018, 225, 84–90. [Google Scholar] [CrossRef]
- Wang, H.; Xu, C.; Cheng, F.; Zhang, M.; Wang, S. Pd/Pt core–shell nanowire arrays as highly effective electrocatalysts for methanol electrooxidation in direct methanol fuel cells. Electrochem. Commun. 2008, 10, 1575–1578. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, C.L.; Lai, Y.S. The enhancement of platinum surface area by alumina template assistance in Sn/Pt core–shell nano/sub-micron sphere structure. Ceram. Int. 2013, 39, 4369–4375. [Google Scholar] [CrossRef]
- Óvári, L.; Berkó, A.; Balázs, N.; Majzik, Z.; Kiss, J. Formation of Rh− Au core− shell nanoparticles on TiO2 (110) surface studied by STM and LEIS. Langmuir 2010, 26, 2167–2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kukovecz, Á.; Pótári, G.; Oszkó, A.; Kónya, Z.; Erdőhelyi, A.; Kiss, J. Probing the interaction of Au, Rh and bimetallic Au–Rh clusters with the TiO2 nanowire and nanotube support. Surf. Sci. 2011, 605, 1048–1055. [Google Scholar] [CrossRef]
- Patel, D.A.; Kress, P.L.; Cramer, L.A.; Larson, A.M.; Sykes, E.C.H. Elucidating the composition of PtAg surface alloys with atomic-scale imaging and spectroscopy. J. Chem. Phys. 2019, 151, 164705. [Google Scholar] [CrossRef] [PubMed]
- Devrim, Y.; Arıca, E.D. Investigation of the effect of graphitized carbon nanotube catalyst support for high temperature PEM fuel cells. Int. J. Hydrog. Energy 2020, 45, 3609–3617. [Google Scholar] [CrossRef]
- Mardle, P.; Ji, X.; Wu, J.; Guan, S.; Dong, H.; Du, S. Thin film electrodes from Pt nanorods supported on aligned N-CNTs for proton exchange membrane fuel cells. Appl. Catal. B Environ. 2020, 260, 118031. [Google Scholar] [CrossRef]
- Bharti, A.; Cheruvally, G.; Muliankeezhu, S. Microwave assisted, facile synthesis of Pt/CNT catalyst for proton exchange membrane fuel cell application. Int. J. Hydrog. Energy 2017, 42, 11622–11631. [Google Scholar] [CrossRef]
- Choi, H.C.; Shim, M.; Bangsaruntip, S.; Dai, H. Spontaneous reduction of metal ions on the sidewalls of carbon nanotubes. J. Am. Chem. Soc. 2002, 124, 9058–9059. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Yang, G.; Wei, S.; Mavinakuli, P.; Guo, Z. Cyclic voltammetric preparation of palladium nanoparticles for ethanol oxidation reaction. Ind. Eng. Chem. Res. 2010, 49, 11415–11420. [Google Scholar] [CrossRef]
- Kim, K.K.; Bae, J.J.; Park, H.K.; Kim, S.M.; Geng, H.-Z.; Park, K.A.; Shin, H.-J.; Yoon, S.-M.; Benayad, A.; Choi, J.-Y. Fermi level engineering of single-walled carbon nanotubes by AuCl3 doping. J. Am. Chem. Soc. 2008, 130, 12757–12761. [Google Scholar] [CrossRef]
- Zheng, M.; Diner, B.A. Solution redox chemistry of carbon nanotubes. J. Am. Chem. Soc. 2004, 126, 15490–15494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jin, M.; Wang, J.; Kim, M.J.; Yang, D.; Xia, Y. Nanocrystals composed of alternating shells of Pd and Pt can be obtained by sequentially adding different precursors. J. Am. Chem. Soc. 2011, 133, 10422–10425. [Google Scholar] [CrossRef]
- Hong, J.W.; Kim, D.; Lee, Y.W.; Kim, M.; Kang, S.W.; Han, S.W. Atomic-distribution-dependent electrocatalytic activity of Au–Pd bimetallic nanocrystals. Angew. Chem. Int. Ed. 2011, 50, 8876–8880. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Jiang, M.; Camargo, P.H.; Cho, E.C.; Tao, J.; Lu, X.; Zhu, Y.; Xia, Y. Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction. Science 2009, 324, 1302–1305. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Yang, H. Synthesis and oxygen reduction electrocatalytic property of Pt-on-Pd bimetallic heteronanostructures. J. Am. Chem. Soc. 2009, 131, 7542–7543. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Zhou, Z.; Zhuang, J.; Wang, X. Pd–Pt random alloy nanocubes with tunable compositions and their enhanced electrocatalytic activities. Chem. Commun. 2010, 46, 1491–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.-N.; Zhang, X.-T.; Wang, Z.-H.; Guo, S.; Li, Y.-J. Cubic superstructures composed of PtPd alloy nanocubes and their enhanced electrocatalysis for methanol oxidation. Chem. Commun. 2016, 52, 12737–12740. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-N.; He, L.-L.; Chen, C.; Wang, A.-J.; Ma, K.-F.; Feng, J.-J. One-pot synthesis of platinum3cobalt nanoflowers with enhanced oxygen reduction and methanol oxidation. J. Power Sour. 2014, 268, 744–751. [Google Scholar] [CrossRef]









| Solution | pH |
|---|---|
| Milli-Q water | 5.62 |
| CNTs + Milli-Q water | 5.32 |
| K2PdCl4 | 4.21 |
| CNTs + K2PdCl4 | 3.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, F.; Kwong, T.-L.; Yung, K.-F. Surfactant-Free Monodispersed Pd Nanoparticles Template for Core-Shell Pd@PdPt Nanoparticles as Electrocatalyst towards Methanol Oxidation Reaction (MOR). Nanomaterials 2022, 12, 260. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12020260
Zheng F, Kwong T-L, Yung K-F. Surfactant-Free Monodispersed Pd Nanoparticles Template for Core-Shell Pd@PdPt Nanoparticles as Electrocatalyst towards Methanol Oxidation Reaction (MOR). Nanomaterials. 2022; 12(2):260. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12020260
Chicago/Turabian StyleZheng, Fulin, Tsz-Lung Kwong, and Ka-Fu Yung. 2022. "Surfactant-Free Monodispersed Pd Nanoparticles Template for Core-Shell Pd@PdPt Nanoparticles as Electrocatalyst towards Methanol Oxidation Reaction (MOR)" Nanomaterials 12, no. 2: 260. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12020260