Biomedical Applications of Metal−Organic Frameworks for Disease Diagnosis and Drug Delivery: A Review
Abstract
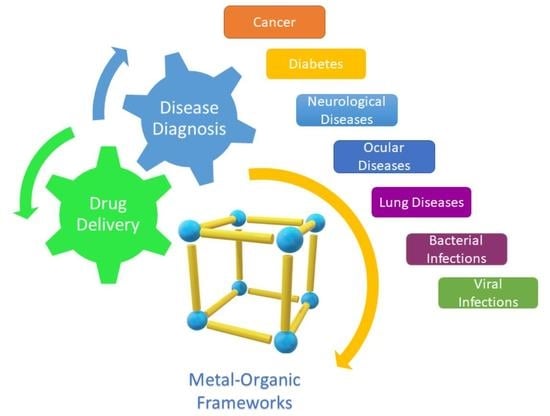
:1. Introduction
2. Metal−Organic Frameworks (MOFs)
2.1. Synthesis of MOFs
2.1.1. Diffusion Method
2.1.2. Electrochemical Method
2.1.3. Microwave-Assisted Method
2.1.4. Mechanochemical Method
2.1.5. Solvothermal Method
2.1.6. Sonochemical Method
2.1.7. Room Temperature Method
2.2. Biomedical Applications of MOFs
2.3. Stimuli-Responsive Therapeutic Platform Based on MOFs
2.4. Toxicity of MOFs in Biological Systems
| Type of Triggers | Examples | Examples of MOFs | Drugs and applications | Remarks | Reference |
|---|---|---|---|---|---|
| Internal | pH | Hollow mesoporous silica at a zeolitic imidazolate framework (HMS@ZIF) | Doxorubicin (DOX), anticancer therapy | Engineer a system that can utilize the pH differences between the blood and the diseased cells to enable drug delivery to chosen sites. | [151,152,153,154] |
| MIL-100(Fe) | Camptothecin (CPT), anticancer therapy | ||||
| ZIF-8 | D-α-Tocopherol succinate (α-TOS), antitumor therapy | ||||
| Temperature | Zinc MOF constructed by semirigid 5-(4′-carboxyphenoxy) nicotinic acid (Zn-cpon-1) | 5-fluorouracil (5-FU), anticancer therapy | Design a delivery system that will merely release the drug at temperatures beyond 37 °C. | [155,156,157,158] | |
| Zinc glycolate MOF (Zn-GA) | Methotrexate (MTX), anticancer treatment | ||||
| UiO-66 | 5-Fu, chemophotothermal therapy | ||||
| Redox potential (Glutathione (GSH) concentration) | Zinc-based 4,4′-dithiobisbenzoic acid MOF (MOF-Zr (DTBA)) | Curcumin (CCM), anticancer therapy | Exploit the concentration gradient between normal and diseased cells, and between intracellular and extracellular environments for targeted drug delivery to certain sites. | [159,160,161,162] | |
| Cyclodextrin MOFs (CD-MOFs) | DOX, anticancer therapy | ||||
| Zr-MOF | Cisplatin, anticancer therapy | ||||
| Enzyme concentration | Porphyrinic MOF (PCN-224) | DOX, anticancer therapy | Design a system by incorporating a certain peptide sequence or chemical bond that is prone to be attacked by disease-related enzymes. | [143,163,164] | |
| UiO-68 | CPT, anticancer therapy | ||||
| External | Ultrasound | NH2-Fe-BDC | DOX, anticancer therapy | Apply local sonication after the injection of encapsulated drugs for targeted delivery purposes. This enables the uniform distribution of micelles and the drug across the pathological cell. | [142,165,166] |
| Fe-NDC | Calcein and DOX, anticancer therapy | ||||
| Magnetic Field | HKUST-1 | Nimesulide, anticancer treatment | After administration, the drug immobilized magnetite carrier can pile up at the targeted site under the course of an external magnetic field. | [167,168,169,170] | |
| PD/M-NMOF | DOX & MB, anticancer treatment | ||||
| ZIF-8@ABFs | RhB | ||||
| Light | o-NBA@ZIF-8 | rifampicin (RFP), bacterial infection therapy | Design a light-sensitive system that goes through reverse disruption under the action of light to enable external control of drug release. | [171,172,173,174] | |
| UiO-AZB | 5-FU, anticancer therapy | ||||
| AuNR@MOFs | CPT, anticancer therapy | ||||
| Heat | CP5-capped UiO-66-NH-Q | 5-FU, treatment of central nervous system diseases | Apply an external heat source to raise the temperature of the cellular environment. | [155,175,176] | |
| CP5-capped UiO-66-NH-A | 5-FU, anticancer therapy |
3. Applications of MOFs for Disease Diagnosis and Drug Delivery
3.1. Cancer
3.2. Diabetes
3.2.1. Anti-Diabetic Agents
3.2.2. Wound Healing
3.3. Neurological Diseases
3.4. Ocular Diseases
3.5. Lung Diseases
3.6. Bacterial Infections
3.7. Viral Infections
3.8. Miscellaneous Diseases
4. Challenges and Future Directions
5. Conclusions
Funding
Conflicts of Interest
References
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Zhang, X.; Lu, Q.; Fei, Z.; Dyson, P.J. Single walled carbon nanotubes as drug delivery vehicles: Targeting doxorubicin to tumors. Biomaterials 2012, 33, 1689–1698. [Google Scholar] [CrossRef]
- Chamundeeswari, M.; Jeslin, J.; Verma, M.L. Nanocarriers for drug delivery applications. Environ. Chem. Lett. 2018, 17, 849–865. [Google Scholar] [CrossRef]
- Erttmann, R.; Erb, N.; Steinhoff, A.; Landbeck, G. Pharmacokinetics of doxorubicin in man: Dose and schedule dependence. J. Cancer Res. Clin. Oncol. 1988, 114, 509–513. [Google Scholar] [CrossRef]
- Shan, K. Anthracycline-Induced Cardiotoxicity. Ann. Intern. Med. 1996, 125, 47. [Google Scholar] [CrossRef]
- Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [Green Version]
- Orellana-Tavra, C.; Baxter, E.F.; Tian, T.; Bennett, T.D.; Slater, N.K.H.; Cheetham, A.K.; Fairen-Jimenez, D. Amorphous metal–organic frameworks for drug delivery. Chem. Commun. 2015, 51, 13878–13881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinho, N.; Damgé, C.; Reis, C.P. Recent Advances in Drug Delivery Systems. J. Biomater. Nanobiotechnol. 2011, 02, 510–526. [Google Scholar] [CrossRef] [Green Version]
- Baskar, G.; Bikku George, G.; Chamundeeswari, M. Synthesis and Characterization of Asparaginase Bound Silver Nanocomposite Against Ovarian Cancer Cell Line A2780 and Lung Cancer Cell Line A549. J. Inorg. Organomet. Polym. Mater. 2016, 27, 87–94. [Google Scholar] [CrossRef]
- Baskar, G.; Lalitha, K.; Garrick, B.G.; Chamundeeswari, M. Conjugation, Labeling and Characterization of Asparaginase Bound Silver Nanoparticles for Anticancer Applications; NISCAIR-CSIR: New Delhi, India, 2017; Volume 55. [Google Scholar]
- Muthukumar, T.; Chamundeeswari, M.; Prabhavathi, S.; Gurunathan, B.; Chandhuru, J.; Sastry, T.P. Carbon nanoparticle from a natural source fabricated for folate receptor targeting, imaging and drug delivery application in A549 lung cancer cells. Eur. J. Pharm. Biopharm. 2014, 88, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Vauthier, C.; Labarre, D.; Ponchel, G. Design aspects of poly(alkylcyanoacrylate) nanoparticles for drug delivery. J. Drug Target. 2007, 15, 641–663. [Google Scholar] [CrossRef] [PubMed]
- Chamundeeswari, M.; Sastry, T.P.; Lakhsmi, B.S.; Senthil, V.; Agostinelli, E. Iron nanoparticles from animal blood for cellular imaging and targeted delivery for cancer treatment. Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 3005–3010. [Google Scholar] [CrossRef] [PubMed]
- Gelperina, S.; Kisich, K.; Iseman, M.D.; Heifets, L. The Potential Advantages of Nanoparticle Drug Delivery Systems in Chemotherapy of Tuberculosis. Am. J. Respir. Crit. Care Med. 2005, 172, 1487–1490. [Google Scholar] [CrossRef] [Green Version]
- Baskar, G.; Garrick, B.G.; Lalitha, K.; Chamundeeswari, M. Gold nanoparticle mediated delivery of fungal asparaginase against cancer cells. J. Drug Deliv. Sci. Technol. 2018, 44, 498–504. [Google Scholar] [CrossRef]
- Rebekah, A.; Sivaselvam, S.; Viswanathan, C.; Prabhu, D.; Gautam, R.; Ponpandian, N. Magnetic nanoparticle-decorated graphene oxide-chitosan composite as an efficient nanocarrier for protein delivery. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125913. [Google Scholar] [CrossRef]
- Luzuriaga, M.A.; Welch, R.P.; Dharmarwardana, M.; Benjamin, C.E.; Li, S.; Shahrivarkevishahi, A.; Popal, S.; Tuong, L.H.; Creswell, C.T.; Gassensmith, J.J. Enhanced Stability and Controlled Delivery of MOF-Encapsulated Vaccines and Their Immunogenic Response In Vivo. ACS Appl. Mater. Interfaces 2019, 11, 9740–9746. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Lemieux, P.; Vinogradov, S.; Alakhov, V. Pluronic® block copolymers: Novel functional molecules for gene therapy. Adv. Drug Deliv. Rev. 2002, 54, 223–233. [Google Scholar] [CrossRef]
- Verma, M.L.; Barrow, C.J.; Puri, M. Nanobiotechnology as a novel paradigm for enzyme immobilisation and stabilisation with potential applications in biodiesel production. Appl. Microbiol. Biotechnol. 2012, 97, 23–39. [Google Scholar] [CrossRef]
- Neubert, R.H.H. Potentials of new nanocarriers for dermal and transdermal drug delivery. Eur. J. Pharm. Biopharm. 2011, 77, 1–2. [Google Scholar] [CrossRef]
- Jahangirian, H.; Ghasemian Lemraski, E.; Webster, T.J.; Rafiee-Moghaddam, R.; Abdollahi, Y. A review of drug delivery systems based on nanotechnology and green chemistry: Green nanomedicine. Int. J. Nanomed. 2017, 12, 2957–2978. [Google Scholar] [CrossRef] [Green Version]
- de Villiers, M.M.; Aramwit, P.; Kwon, G.S. Nanotechnology in Drug Delivery; de Villiers, M.M., Aramwit, P., Kwon, G.S., Eds.; Springer: New York, NY, USA, 2009; Volume 10. [Google Scholar]
- Mirza, A.Z.; Siddiqui, F.A. Nanomedicine and drug delivery: A mini review. Int. Nano Lett. 2014, 4, 94. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Wang, J.; Wang, T.; Zhong, J.; Bao, Y.; Hao, H. Recent Progress on Nanostructures for Drug Delivery Applications. J. Nanomater. 2016, 2016, 5762431. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Khan, A.I.; Cai, X.; Song, Y.; Lyu, Z.; Du, D.; Dutta, P.; Lin, Y. Overcoming blood–brain barrier transport: Advances in nanoparticle-based drug delivery strategies. Mater. Today 2020, 37, 112–125. [Google Scholar] [CrossRef]
- López-Dávila, V.; Seifalian, A.M.; Loizidou, M. Organic nanocarriers for cancer drug delivery. Curr. Opin. Pharmacol. 2012, 12, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.A.; Bimbo, L.M.; Peltonen, L.; Hirvonen, J. Inorganic Nanoparticles in Targeted Drug Delivery and Imaging. In Targeted Drug Delivery: Concepts and Design; Springer: Cham, Switzerland, 2014; pp. 571–613. [Google Scholar] [CrossRef]
- Karami, A.; Mohamed, O.; Ahmed, A.; Husseini, G.A.; Sabouni, R. Recent Advances in Metal-Organic Frameworks as Anticancer Drug Delivery Systems: A Review. Anti-Cancer Agents Med. Chem. 2021, 21, 2487–2504. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.Y.; Sun, D.M.; Zhu, R.R.; Du, X.L.; Liu, H.; Wang, S.L. pH-sensitive strontium carbonate nanoparticles as new anticancer vehicles for controlled etoposide release. Int. J. Nanomed. 2012, 7, 5781. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angew. Chem. Int. Ed. 2014, 126, 12520–12568. [Google Scholar] [CrossRef]
- Mishra, B.; Patel, B.B.; Tiwari, S. Colloidal nanocarriers: A review on formulation technology, types and applications toward targeted drug delivery. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Ochekpe, N.A.; Olorunfemi, P.O.; Ngwuluka, N.C. Nanotechnology and Drug Delivery Part 2: Nanostructures for Drug Delivery. Trop. J. Pharm. Res. 2009, 8. [Google Scholar] [CrossRef]
- Meng, F.; Zhong, Z.; Feijen, J. Stimuli-Responsive Polymersomes for Programmed Drug Delivery. Biomacromolecules 2009, 10, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Cho, E.C.; Shim, J.; Kim, D.-H.; An, E.J.; Kim, J. Polymer-associated liposomes as a novel delivery system for cyclodextrin-bound drugs. J. Colloid Interface Sci. 2008, 320, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Korting, H.C.; Schäfer-Korting, M. Carriers in the Topical Treatment of Skin Disease. Drug Deliv. 2009, 197, 435–468. [Google Scholar] [CrossRef]
- Schaffazick, S.R.; Pohlmann, A.R.; de Cordova, C.A.S.; Creczynski-Pasa, T.B.; Guterres, S.S. Protective properties of melatonin-loaded nanoparticles against lipid peroxidation. Int. J. Pharm. 2005, 289, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Castro, G.A.; Oréfice, R.L.; Vilela, J.M.C.; Andrade, M.S.; Ferreira, L.A.M. Development of a new solid lipid nanoparticle formulation containing retinoic acid for topical treatment of acne. J. Microencapsul. 2007, 24, 395–407. [Google Scholar] [CrossRef]
- Huda, S.; Alam, M.A.; Sharma, P.K. Smart nanocarriers-based drug delivery for cancer therapy: An innovative and developing strategy. J. Drug Deliv. Sci. Technol. 2020, 60, 102018. [Google Scholar] [CrossRef]
- Bhatia, S. Nanoparticles types, classification, characterization, fabrication methods and drug delivery applications. In Natural Polymer Drug Delivery Systems; Springer: Cham, Switzerland, 2016; pp. 33–93. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Geißler, D.; Charbonnière, L.J.; Ziessel, R.F.; Butlin, N.G.; Löhmannsröben, H.-G.; Hildebrandt, N. Quantum Dot Biosensors for Ultrasensitive Multiplexed Diagnostics. Angew. Chem. Int. Ed. 2010, 49, 1396–1401. [Google Scholar] [CrossRef]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.-M.; Cui, Y.; Zhou, W.; Qian, G.; Chen, B. Emerging Multifunctional Metal-Organic Framework Materials. Adv. Mater. 2016, 28, 8819–8860. [Google Scholar] [CrossRef]
- Nandasiri, M.I.; Jambovane, S.R.; McGrail, B.P.; Schaef, H.T.; Nune, S.K. Adsorption, separation, and catalytic properties of densified metal-organic frameworks. Coord. Chem. Rev. 2016, 311, 38–52. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Pei, X.; Gao, H.; Chen, S.; Wang, J. Metal-organic framework-based nanomaterials for biomedical applications. Chin. Chem. Lett. 2020, 31, 1060–1070. [Google Scholar] [CrossRef]
- Eddaoudi, M. Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.-W. Hydrogen Storage in Metal–Organic Frameworks. Chem. Rev. 2011, 112, 782–835. [Google Scholar] [CrossRef]
- Chen, B.; Xiang, S.; Qian, G. Metal−Organic Frameworks with Functional Pores for Recognition of Small Molecules. Acc. Chem. Res. 2010, 43, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huo, F. Metal–organic framework composites: From fundamentals to applications. Nanoscale 2015, 7, 7482–7501. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Yan, L.; Wang, G.; Liu, A. Recent advances in the synthesis of spherical and nanoMOF-derived multifunctional porous carbon for nanomedicine applications. Coord. Chem. Rev. 2019, 391, 69–89. [Google Scholar] [CrossRef]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal–organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Peters, A.W.; Platero-Prats, A.E.; Liu, J.; Kung, C.-W.; Noh, H.; DeStefano, M.R.; Schweitzer, N.M.; Chapman, K.W.; Hupp, J.T.; et al. Fine-Tuning the Activity of Metal–Organic Framework-Supported Cobalt Catalysts for the Oxidative Dehydrogenation of Propane. J. Am. Chem. Soc. 2017, 139, 15251–15258. [Google Scholar] [CrossRef]
- Al Sharabati, M.; Sabouni, R. Selective removal of dual dyes from aqueous solutions using a metal organic framework (MIL-53(Al)). Polyhedron 2020, 190, 114762. [Google Scholar] [CrossRef]
- Schoedel, A.; Ji, Z.; Yaghi, O.M. The role of metal–organic frameworks in a carbon-neutral energy cycle. Nat. Energy 2016, 1. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, W. Metal–organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2014, 43, 5982–5993. [Google Scholar] [CrossRef]
- Lian, X.; Fang, Y.; Joseph, E.; Wang, Q.; Li, J.; Banerjee, S.; Lollar, C.; Wang, X.; Zhou, H.-C. Enzyme–MOF (metal–organic framework) composites. Chem. Soc. Rev. 2017, 46, 3386–3401. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wang, K.Y.; Lv, X.L.; Yan, T.H.; Zhou, H.C. Hierarchically porous metal-organic frameworks: Synthetic strategies and applications. Natl. Sci. Rev. 2020, 7, 1743–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, H.; Li, M.; Gao, J.; Zhang, Z.; Ma, S.; Chen, Y. Incorporation of biomolecules in Metal-Organic Frameworks for advanced applications. Coord. Chem. Rev. 2019, 384, 90–106. [Google Scholar] [CrossRef]
- Malik, P.; Gupta, R.; Ameta, R.K. Unique attributes of metal-organic frameworks in drug delivery. In Metal-Organic Frameworks for Chemical Reactions; Elsevier: Amsterdam, The Netherlands, 2021; pp. 389–415. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, L.; Yang, Y.; Qian, X.; Fu, T.; Li, X.; Yang, Z.; Yan, H.; Cui, C.; Tan, W. Metal–Organic Framework Nanocarriers for Drug Delivery in Biomedical Applications. Nano-Micro Lett. 2020, 12. [Google Scholar] [CrossRef]
- Anand, R.; Borghi, F.; Manoli, F.; Manet, I.; Agostoni, V.; Reschiglian, P.; Gref, R.; Monti, S. Host–Guest Interactions in Fe(III)-Trimesate MOF Nanoparticles Loaded with Doxorubicin. J. Phys. Chem. B 2014, 118, 8532–8539. [Google Scholar] [CrossRef] [PubMed]
- Bunzen, H.; Grzywa, M.; Hambach, M.; Spirkl, S.; Volkmer, D. From Micro to Nano: A Toolbox for Tuning Crystal Size and Morphology of Benzotriazolate-Based Metal-Organic Frameworks. Cryst. Growth Des. 2016, 16, 3190–3197. [Google Scholar] [CrossRef]
- Schwirn, K.; Tietjen, L.; Beer, I. Why are nanomaterials different and how can they be appropriately regulated under REACH? Environ. Sci. Eur. 2014, 26, 4. [Google Scholar] [CrossRef] [Green Version]
- Głowniak, S.; Szczęśniak, B.; Choma, J.; Jaroniec, M. Mechanochemistry: Toward green synthesis of metal–organic frameworks. Mater. Today 2021, 46, 109–124. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Kim, J.; Ahn, W.-S. Synthesis of metal-organic frameworks: A mini review. Korean J. Chem. Eng. 2013, 30, 1667–1680. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Zhao, B.; Shi, W.; Xia, J.; Cheng, P.; Liao, D.-Z.; Yan, S.-P.; Jiang, Z.-H. Microporous Metal−Organic Frameworks Built on a Ln3Cluster as a Six-Connecting Node. Chem. Mater. 2005, 17, 2866–2874. [Google Scholar] [CrossRef]
- Wang, D.; He, H.; Chen, X.; Feng, S.; Niu, Y.; Sun, D. A 3D porous metal–organic framework constructed of 1D zigzag and helical chains exhibiting selective anion exchange. CrystEngComm 2010, 12, 1041–1043. [Google Scholar] [CrossRef]
- Mueller, U.; Schubert, M.; Teich, F.; Puetter, H.; Schierle-Arndt, K.; Pastré, J. Metal–organic frameworks—prospective industrial applications. J. Mater. Chem. 2006, 16, 626–636. [Google Scholar] [CrossRef]
- Campagnol, N.; Souza, E.R.; De Vos, D.E.; Binnemans, K.; Fransaer, J. Luminescent terbium-containing metal–organic framework films: New approaches for the electrochemical synthesis and application as detectors for explosives. Chem. Commun. 2014, 50, 12545–12547. [Google Scholar] [CrossRef] [Green Version]
- Phang, W.J.; Lee, W.R.; Yoo, K.; Ryu, D.W.; Kim, B.; Hong, C.S. pH-Dependent Proton Conducting Behavior in a Metal-Organic Framework Material. Angew. Chem. Int. Ed. 2014, 53, 8383–8387. [Google Scholar] [CrossRef]
- Sabouni, R.; Kazemian, H.; Rohani, S. Microwave Synthesis of the CPM-5 Metal Organic Framework. Chem. Eng. Technol. 2012, 35, 1085–1092. [Google Scholar] [CrossRef]
- Babu, R.; Roshan, R.; Kathalikkattil, A.C.; Kim, D.W.; Park, D.W. Rapid, Microwave-Assisted Synthesis of Cubic, Three-Dimensional, Highly Porous MOF-205 for Room Temperature CO2 Fixation via Cyclic Carbonate Synthesis. ACS Appl. Mater. Interfaces 2016, 8, 33723–33731. [Google Scholar] [CrossRef]
- Pichon, A.; Lazuen-Garay, A.; James, S.L. Solvent-free synthesis of a microporous metal–organic framework. CrystEngComm 2006, 8, 211. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Morsali, A.; Junk, P.C. Rapid mechanochemical synthesis of two new Cd(ii)-based metal–organic frameworks with high removal efficiency of Congo red. CrystEngComm 2015, 17, 686–692. [Google Scholar] [CrossRef]
- Qiu, S.; Zhu, G. Molecular engineering for synthesizing novel structures of metal–organic frameworks with multifunctional properties. Coord. Chem. Rev. 2009, 253, 2891–2911. [Google Scholar] [CrossRef]
- Shen, L.; Wu, W.; Liang, R.; Lin, R.; Wu, L. Highly dispersed palladium nanoparticles anchored on UiO-66(NH2) metal-organic framework as a reusable and dual functional visible-light-driven photocatalyst. Nanoscale 2013, 5, 9374. [Google Scholar] [CrossRef]
- Morsali, A.; Monfared, H.H.; Morsali, A.; Janiak, C. Ultrasonic irradiation assisted syntheses of one-dimensional di(azido)-dipyridylamine Cu(II) coordination polymer nanoparticles. Ultrason. Sonochem. 2015, 23, 208–211. [Google Scholar] [CrossRef]
- Son, W.-J.; Kim, J.J.; Kim, J.J.; Ahn, W.-S. Sonochemical synthesis of MOF-5. Chem. Commun. 2008, 47, 6336. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.-W.; Yang, D.-A.; Kim, J.; Kim, J.; Ahn, W.-S. Facile synthesis of MOF-177 by a sonochemical method using 1-methyl-2-pyrrolidinone as a solvent. Dalt. Trans. 2010, 39, 2883. [Google Scholar] [CrossRef]
- Li, Z.Q.; Qiu, L.G.; Xu, T.; Wu, Y.; Wang, W.; Wu, Z.Y.; Jiang, X. Ultrasonic synthesis of the microporous metal–organic framework Cu3(BTC)2 at ambient temperature and pressure: An efficient and environmentally friendly method. Mater. Lett. 2009, 63, 78–80. [Google Scholar] [CrossRef]
- Abazari, R.; Mahjoub, A.R.; Shariati, J. Synthesis of a nanostructured pillar MOF with high adsorption capacity towards antibiotics pollutants from aqueous solution. J. Hazard. Mater. 2019, 366, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiari, N.; Azizian, S. Nanoporous Carbon Derived from MOF-5: A Superadsorbent for Copper Ions. ACS Omega 2018, 3, 16954–16959. [Google Scholar] [CrossRef] [Green Version]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A review on metal-organic frameworks: Synthesis and applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Mueller, U.; Puetter, H.; Hesse, M.; Schubert, M.; Wessel, H.; Huff, J.; Guzmann, M. Method for Electrochemical Production of a Crystalline Porous Metal Organic Skeleton Material. U.S. Patent No. 7,968,739, 28 June 2011. [Google Scholar]
- Sun, Y.; Zhou, H.-C. Recent progress in the synthesis of metal–organic frameworks. Sci. Technol. Adv. Mater. 2015, 16, 54202. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2011, 112, 933–969. [Google Scholar] [CrossRef]
- Pirzadeh, K.; Ghoreyshi, A.A.; Rahimnejad, M.; Mohammadi, M. Electrochemical synthesis, characterization and application of a microstructure Cu3(BTC)2 metal organic framework for CO2 and CH4 separation. Korean J. Chem. Eng. 2018, 35, 974–983. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, T.; Zou, X.; Liang, X.; Zhu, G.; Qu, F. Electrochemical synthesis of metal organic framework films with proton conductive property. Solid State Ion. 2017, 301, 125–132. [Google Scholar] [CrossRef]
- Saeed, T.; Naeem, A.; Ud Din, I.; Alotaibi, M.A.; Alharthi, A.I.; Wali Khan, I.; Huma Khan, N.; Malik, T. Structure, nomenclature and viable synthesis of micro/nanoscale metal organic frameworks and their remarkable applications in adsorption of organic pollutants. Microchem. J. 2020, 159, 105579. [Google Scholar] [CrossRef]
- YANG, H.; LIU, X.; SONG, X.; YANG, T.; LIANG, Z.; FAN, C. In situ electrochemical synthesis of MOF-5 and its application in improving photocatalytic activity of BiOBr. Trans. Nonferrous Met. Soc. China 2015, 25, 3987–3994. [Google Scholar] [CrossRef]
- de Lima Neto, O.J.; de Oliveira Frós, A.C.; Barros, B.S.; de Farias Monteiro, A.F.; Kulesza, J. Rapid and efficient electrochemical synthesis of a zinc-based nano-MOF for Ibuprofen adsorption. New J. Chem. 2019, 43, 5518–5524. [Google Scholar] [CrossRef]
- Yang, H.; Du, H.; Zhang, L.; Liang, Z.; Li, W. Electrosynthesis and Electrochemical Mechanism of Zn-Based Metal-Organic Frameworks. Int. J. Electrochem. Sci. 2015, 10, 1420–1433. [Google Scholar]
- Jin, T.; Hwang, Y.K.; Hong, D.-Y.; Jhung, S.H.; Hwang, J.-S.; Park, S.-E.; Kim, Y.H.; Chang, J.-S. Microwave synthesis, characterization and catalytic properties of titanium-incorporated ZSM-5 zeolite. Res. Chem. Intermed. 2007, 33, 501–512. [Google Scholar] [CrossRef]
- Tompsett, G.A.; Conner, W.C.; Yngvesson, K.S. Microwave synthesis of nanoporous materials. ChemPhysChem 2006, 7, 296–319. [Google Scholar] [CrossRef] [PubMed]
- Klinowski, J.; Almeida Paz, F.A.; Silva, P.; Rocha, J. Microwave-Assisted Synthesis of Metal–Organic Frameworks. Dalt. Trans. 2011, 40, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Amo-Ochoa, P.; Givaja, G.; Miguel, P.J.S.; Castillo, O.; Zamora, F. Microwave assisted hydrothermal synthesis of a novel CuI-sulfate-pyrazine MOF. Inorg. Chem. Commun. 2007, 10, 921–924. [Google Scholar] [CrossRef]
- Park, S.-E.; Chang, J.-S.; Hwang, Y.K.; Kim, D.S.; Jhung, S.H.; Hwang, J.S. Supramolecular Interactions and Morphology Control in Microwave Synthesis of Nanoporous Materials. Catal. Surv. Asia 2004, 8, 91–110. [Google Scholar] [CrossRef]
- Hwang, Y.K.; Chang, J.-S.; Park, S.-E.; Kim, D.S.; Kwon, Y.-U.; Jhung, S.H.; Hwang, J.-S.; Park, M.S. Microwave Fabrication of MFI Zeolite Crystals with a Fibrous Morphology and Their Applications. Angew. Chem. Int. Ed. 2005, 44, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Trong On, D.; Nguyen, S.V.; Hulea, V.; Dumitriu, E.; Kaliaguine, S. Mono- and bifunctional MFI, BEA and MCM-41 titanium-molecular sieves. Part 1. Synthesis and characterization. Microporous Mesoporous Mater. 2003, 57, 169–180. [Google Scholar] [CrossRef]
- Kang, K.K.; Park, C.H.; Ahn, W.S. Microwave preparation of a titanium-substituted mesoporous molecular sieve. Catal. Lett. 1999, 59, 45–49. [Google Scholar] [CrossRef]
- Kaupp, G. Mechanochemistry: The varied applications of mechanical bond-breaking. CrystEngComm 2009, 11, 388–403. [Google Scholar] [CrossRef]
- Garay, A.L.; Pichon, A.; James, S.L. Solvent-free synthesis of metal complexes. Chem. Soc. Rev. 2007, 36, 846. [Google Scholar] [CrossRef]
- George, P.; Das, R.K.; Chowdhury, P. Facile microwave synthesis of Ca-BDC metal organic framework for adsorption and controlled release of Curcumin. Microporous Mesoporous Mater. 2019, 281, 161–171. [Google Scholar] [CrossRef]
- Xu, H.; Zeiger, B.W.; Suslick, K.S. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013, 42, 2555–2567. [Google Scholar] [CrossRef] [Green Version]
- Dai, S.; Nouar, F.; Zhang, S.; Tissot, A.; Serre, C. One-Step Room-Temperature Synthesis of Metal(IV) Carboxylate Metal—Organic Frameworks. Angew. Chem. Int. Ed. 2021, 60, 4282–4288. [Google Scholar] [CrossRef]
- Getachew, N.; Chebude, Y.; Diaz, I.; Sanchez-Sanchez, M. Room temperature synthesis of metal organic framework MOF-2. J. Porous Mater. 2014, 21, 769–773. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Hunt, J.R.; Yaghi, O.M. Room temperature synthesis of metal-organic frameworks: MOF-5, MOF-74, MOF-177, MOF-199, and IRMOF-0. Tetrahedron 2008, 64, 8553–8557. [Google Scholar] [CrossRef]
- Bang, J.H.; Suslick, K.S. Applications of Ultrasound to the Synthesis of Nanostructured Materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ayyoubzadeh, S.M.; Ghorbani-Bidkorbeh, F.; Shahhosseini, S.; Dadashzadeh, S.; Asadian, E.; Mosayebnia, M.; Siavashy, S. An investigation of affecting factors on MOF characteristics for biomedical applications: A systematic review. Heliyon 2021, 7, e06914. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Ihsanullah. Toxicity of nanoscale metal-organic frameworks in biological systems. In Metal-Organic Frameworks for Biomedical Applications; Woodhead Publishing: Sawston, UK, 2020; pp. 383–395. [Google Scholar] [CrossRef]
- Simon-Yarza, T.; Mielcarek, A.; Couvreur, P.; Serre, C. Nanoparticles of Metal-Organic Frameworks: On the Road to In Vivo Efficacy in Biomedicine. Adv. Mater. 2018, 30, 1707365. [Google Scholar] [CrossRef]
- Sun, C.Y.; Qin, C.; Wang, C.G.; Su, Z.M.; Wang, S.; Wang, X.L.; Yang, G.S.; Shao, K.Z.; Lan, Y.Q.; Wang, E.B. Chiral Nanoporous Metal-Organic Frameworks with High Porosity as Materials for Drug Delivery. Adv. Mater. 2011, 23, 5629–5632. [Google Scholar] [CrossRef]
- He, S.; Wu, L.; Li, X.; Sun, H.; Xiong, T.; Liu, J.; Huang, C.; Xu, H.; Sun, H.; Chen, W.; et al. Metal-organic frameworks for advanced drug delivery. Acta Pharm. Sin. B 2021, 11, 2362–2395. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Li, L.; Yu, X.L.; Liu, L.; Meng, Q.; Wang, F.; Zhang, R. Functionalization of mixed ligand metal-organic frameworks as the transport vehicles for drugs. J. Colloid Interface Sci. 2017, 486, 128–135. [Google Scholar] [CrossRef]
- Leng, X.; Dong, X.; Wang, W.; Sai, N.; Yang, C.; You, L.; Huang, H.; Yin, X.; Ni, J. Biocompatible Fe-Based Micropore Metal-Organic Frameworks as Sustained-Release Anticancer Drug Carriers. Molecules 2018, 23, 2490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.Y.; Qin, C.; Wang, X.L.; Yang, G.S.; Shao, K.Z.; Lan, Y.Q.; Su, Z.M.; Huang, P.; Wang, C.G.; Wang, E.B. Zeolitic imidazolate framework-8 as efficient pH-sensitive drug delivery vehicle. Dalt. Trans. 2012, 41, 6906–6909. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, Y.; Chi, B.; Lin, C.; Tian, F.; Xu, M.; Wang, Y.; Xu, Z.; Li, L.; Wang, J. Synthesis of ‘dual-key-and-lock’ drug carriers for imaging and improved drug release. Nanotechnology 2020, 31, 445102. [Google Scholar] [CrossRef]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef]
- Fattal, E.; Grabowski, N.; Mura, S.; Vergnaud, J.; Tsapis, N.; Hillaireau, H. Lung Toxicity of Biodegradable Nanoparticles. J. Biomed. Nanotechnol. 2014, 10, 2852–2864. [Google Scholar] [CrossRef]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, S.; Reboul, J.; Diring, S.; Sumida, K.; Kitagawa, S. Structuring of metal–organic frameworks at the mesoscopic/macroscopic scale. Chem. Soc. Rev. 2014, 43, 5700–5734. [Google Scholar] [CrossRef]
- Wang, D.; Jana, D.; Zhao, Y. Metal-Organic Framework Derived Nanozymes in Biomedicine. Acc. Chem. Res. 2020, 53, 1389–1400. [Google Scholar] [CrossRef]
- Wu, Y.; Darland, D.C.; Zhao, J.X. Nanozymes—Hitting the Biosensing “Target”. Sensors 2021, 21, 5201. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Lollar, C.T.; Xiao, Z.; Fang, Y.; Zhou, H.C. Biomedical Integration of Metal–Organic Frameworks. Trends Chem. 2020, 2, 467–479. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, L.; Jiang, K.; He, H.; Yang, Y.; Cui, Y.; Li, B.; Qian, G. Nanoscale fluorescent metal–organic framework composites as a logic platform for potential diagnosis of asthma. Biosens. Bioelectron. 2019, 130, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Hu, Y.; Li, G. Biocompatible Au@Ag nanorod@ZIF-8 core-shell nanoparticles for surface-enhanced Raman scattering imaging and drug delivery. Talanta 2019, 200, 212–217. [Google Scholar] [CrossRef]
- Taylor-Pashow, K.M.L.; Della Rocca, J.; Xie, Z.; Tran, S.; Lin, W. Postsynthetic Modifications of Iron-Carboxylate Nanoscale Metal−Organic Frameworks for Imaging and Drug Delivery. J. Am. Chem. Soc. 2009, 131, 14261–14263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, K.; He, C.; Lin, W. Nanoscale Metal–Organic Framework for Highly Effective Photodynamic Therapy of Resistant Head and Neck Cancer. J. Am. Chem. Soc. 2014, 136, 16712–16715. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Chu, C.; Zhang, Y.; Su, Z.; Lin, H.; Pang, X.; Wang, X.; Liu, G.; Li, W. Self-Assembled Metal-Organic Nanoparticles for Multimodal Imaging-Guided Photothermal Therapy of Hepatocellular Carcinoma. J. Biomed. Nanotechnol. 2018, 14, 1934–1943. [Google Scholar] [CrossRef] [PubMed]
- Kundu, T.; Mitra, S.; Díaz Díaz, D.; Banerjee, R. Gadolinium(III)-Based Porous Luminescent Metal-Organic Frameworks for Bimodal Imaging. Chempluschem 2016, 81, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, L.; Ma, C.; Wang, W.; Ding, J.; Liu, S.; Zhang, X.; Xie, Z. BODIPY-containing nanoscale metal–organic frameworks as contrast agents for computed tomography. J. Mater. Chem. B 2017, 5, 2330–2336. [Google Scholar] [CrossRef]
- Cai, W.; Gao, H.; Chu, C.; Wang, X.; Wang, J.; Zhang, P.; Lin, G.; Li, W.; Liu, G.; Chen, X. Engineering Phototheranostic Nanoscale Metal–Organic Frameworks for Multimodal Imaging-Guided Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 2040–2051. [Google Scholar] [CrossRef]
- Ma, W.; Jiang, Q.; Yu, P.; Yang, L.; Mao, L. Zeolitic Imidazolate Framework-Based Electrochemical Biosensor for in Vivo Electrochemical Measurements. Anal. Chem. 2013, 85, 7550–7557. [Google Scholar] [CrossRef]
- Millward, A.R.; Yaghi, O.M. Metal−Organic Frameworks with Exceptionally High Capacity for Storage of Carbon Dioxide at Room Temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef]
- Xu, R.; Wang, Y.; Duan, X.; Lu, K.; Micheroni, D.; Hu, A.; Lin, W. Nanoscale Metal–Organic Frameworks for Ratiometric Oxygen Sensing in Live Cells. J. Am. Chem. Soc. 2016, 138, 2158–2161. [Google Scholar] [CrossRef] [Green Version]
- deKrafft, K.E.; Boyle, W.S.; Burk, L.M.; Zhou, O.Z.; Lin, W. Zr- and Hf-based nanoscale metal–organic frameworks as contrast agents for computed tomography. J. Mater. Chem. 2012, 22, 18139. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Zhang, J.; Yue, D.; Lian, X.; Cui, Y.; Yang, Y.; Qian, G. A highly sensitive near-infrared luminescent metal–organic framework thermometer in the physiological range. Chem. Commun. 2016, 52, 8259–8262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-W.; Zhang, H.-T.; Du, Z.-Y.; Wang, X.; Yu, S.-H.; Jiang, H.-L. Water-stable metal–organic frameworks with intrinsic peroxidase-like catalytic activity as a colorimetric biosensing platform. Chem. Commun. 2014, 50, 1092–1094. [Google Scholar] [CrossRef]
- Liu, M.; Du, H.; Zhang, W.; Zhai, G. Internal stimuli-responsive nanocarriers for drug delivery: Design strategies and applications. Mater. Sci. Eng. C 2017, 71, 1267–1280. [Google Scholar] [CrossRef]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Li, F.; Baik, S.; Shao, W.; Ling, D.; Hyeon, T. Surface design of magnetic nanoparticles for stimuli-responsive cancer imaging and therapy. Biomaterials 2017, 136, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, J.; Bomba, H.N.; Zhu, Y.; Gu, Z. Mechanical Force-Triggered Drug Delivery. Chem. Rev. 2016, 116, 12536–12563. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Wang, Q.Y.; Chen, K.L.; Luo, J.B.; Zhou, Q.H.; Lin, J. Reduction/temperature/pH multi-stimuli responsive core cross-linked polypeptide hybrid micelles for triggered and intracellular drug release. Colloids Surf. B Biointerfaces 2018, 170, 373–381. [Google Scholar] [CrossRef]
- Liu, J.; Pang, Y.; Zhu, Z.; Wang, D.; Li, C.; Huang, W.; Zhu, X.; Yan, D. Therapeutic nanocarriers with hydrogen peroxide-triggered drug release for cancer treatment. Biomacromolecules 2013, 14, 1627–1636. [Google Scholar] [CrossRef]
- Wang, H.S. Metal–organic frameworks for biosensing and bioimaging applications. Coord. Chem. Rev. 2017, 349, 139–155. [Google Scholar] [CrossRef]
- Wuttke, S.; Zimpel, A.; Bein, T.; Braig, S.; Stoiber, K.; Vollmar, A.; Müller, D.; Haastert-Talini, K.; Schaeske, J.; Stiesch, M.; et al. Validating Metal-Organic Framework Nanoparticles for Their Nanosafety in Diverse Biomedical Applications. Adv. Healthc. Mater. 2017, 6, 1600818. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Anand, B.; Tsang, Y.F.; Kim, K.H.; Khullar, S.; Wang, B. Regeneration, degradation, and toxicity effect of MOFs: Opportunities and challenges. Environ. Res. 2019, 176, 108488. [Google Scholar] [CrossRef]
- Sajid, M. Toxicity of nanoscale metal organic frameworks: A perspective. Environ. Sci. Pollut. Res. 2016, 23, 14805–14807. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Pan, Y.; Sun, Z.; Liu, D.; Xu, H.; Yu, Q.; Trivedi, M.; Kumar, A.; Chen, J.; Liu, J. Design of Metal-Organic Frameworks for pH-Responsive Drug Delivery Application. Mini-Rev. Med. Chem. 2019, 19, 1644–1665. [Google Scholar] [CrossRef]
- Jia, X.; Yang, Z.; Wang, Y.; Chen, Y.; Yuan, H.; Chen, H.; Xu, X.; Gao, X.; Liang, Z.; Sun, Y.; et al. Hollow Mesoporous Silica@Metal–Organic Framework and Applications for pH-Responsive Drug Delivery. ChemMedChem 2018, 13, 400–405. [Google Scholar] [CrossRef]
- Cabrera-García, A.; Checa-Chavarria, E.; Rivero-Buceta, E.; Moreno, V.; Fernández, E.; Botella, P. Amino modified metal-organic frameworks as pH-responsive nanoplatforms for safe delivery of camptothecin. J. Colloid Interface Sci. 2019, 541, 163–174. [Google Scholar] [CrossRef]
- Sun, Q.; Bi, H.; Wang, Z.; Li, C.; Wang, X.; Xu, J.; Zhu, H.; Zhao, R.; He, F.; Gai, S.; et al. Hyaluronic acid-targeted and pH-responsive drug delivery system based on metal-organic frameworks for efficient antitumor therapy. Biomaterials 2019, 223, 119473. [Google Scholar] [CrossRef]
- Pandey, A.; Dhas, N.; Deshmukh, P.; Caro, C.; Patil, P.; Luisa García-Martín, M.; Padya, B.; Nikam, A.; Mehta, T.; Mutalik, S. Heterogeneous surface architectured metal-organic frameworks for cancer therapy, imaging, and biosensing: A state-of-the-art review. Coord. Chem. Rev. 2020, 409, 213212. [Google Scholar] [CrossRef]
- Lin, W.; Cui, Y.; Yang, Y.; Hu, Q.; Qian, G. A biocompatible metal–organic framework as a pH and temperature dual-responsive drug carrier. Dalt. Trans. 2018, 47, 15882–15887. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.; Fan, R.; Wang, F.; Nie, H.; Du, X.; Gai, S.; Wang, P.; Yang, Y. Dual-Stimulus-Triggered Programmable Drug Release and Luminescent Ratiometric pH Sensing from Chemically Stable Biocompatible Zinc Metal–Organic Framework. ACS Appl. Mater. Interfaces 2018, 10, 22746–22756. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-X.; Yan, H.-J.; Gao, J.; Cheng, Y.; Yang, J.; Wu, J.-R.; Gong, B.-J.; Zhang, H.-Y.; Yang, Y.-W. Multifunctional Supramolecular Materials Constructed from Polypyrrole@UiO-66 Nanohybrids and Pillararene Nanovalves for Targeted Chemophotothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 34655–34663. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, D.; Guo, Z. Endogenous Stimuli-responsive Nanocarriers for Drug Delivery. Chem. Lett. 2016, 45, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Lei, B.; Wang, M.; Jiang, Z.; Qi, W.; Su, R.; He, Z. Constructing Redox-Responsive Metal–Organic Framework Nanocarriers for Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 16698–16706. [Google Scholar] [CrossRef]
- Xue, Q.; Ye, C.; Zhang, M.; Hu, X.; Cai, T. Glutathione responsive cubic gel particles cyclodextrin metal-organic frameworks for intracellular drug delivery. J. Colloid Interface Sci. 2019, 551, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gong, C.S.; Dai, Y.; Yang, Z.; Yu, G.; Liu, Y.; Zhang, M.; Lin, L.; Tang, W.; Zhou, Z.; et al. In situ polymerization on nanoscale metal-organic frameworks for enhanced physiological stability and stimulus-responsive intracellular drug delivery. Biomaterials 2019, 218, 119365. [Google Scholar] [CrossRef]
- Kim, K.; Lee, S.; Jin, E.; Palanikumar, L.; Lee, J.H.; Kim, J.C.; Nam, J.S.; Jana, B.; Kwon, T.-H.; Kwak, S.K.; et al. MOF × Biopolymer: Collaborative Combination of Metal–Organic Framework and Biopolymer for Advanced Anticancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 27512–27520. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Luo, G.-F.; Sohn, Y.S.; Nechushtai, R.; Willner, I. Enzyme-Driven Release of Loads from Nucleic Acid–Capped Metal–Organic Framework Nanoparticles. Adv. Funct. Mater. 2019, 29, 1805341. [Google Scholar] [CrossRef]
- Ahmed, A.; Karami, A.; Sabouni, R.; Husseini, G.A.; Paul, V. pH and ultrasound dual-responsive drug delivery system based on PEG–folate-functionalized Iron-based metal–organic framework for targeted doxorubicin delivery. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127062. [Google Scholar] [CrossRef]
- Ibrahim, M.; Sabouni, R.; Husseini, G.A.; Karami, A.; Bai, R.G.; Mukhopadhyay, D. Facile Ultrasound-Triggered Release of Calcein and Doxorubicin from Iron-Based Metal-Organic Frameworks. J. Biomed. Nanotechnol. 2021, 16, 1359–1369. [Google Scholar] [CrossRef]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005, 100, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ke, F.; Yuan, Y.-P.; Qiu, L.-G.; Shen, Y.-H.; Xie, A.-J.; Zhu, J.-F.; Tian, X.-Y.; Zhang, L.-D. Facile fabrication of magnetic metal–organic framework nanocomposites for potential targeted drug delivery. J. Mater. Chem. 2011, 21, 3843–3848. [Google Scholar] [CrossRef]
- Sharma, S.; Sethi, K.; Roy, I. Magnetic nanoscale metal–organic frameworks for magnetically aided drug delivery and photodynamic therapy. New J. Chem. 2017, 41, 11860–11866. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.Z.; Alcântara, C.C.; Sevim, S.; Hoop, M.; Terzopoulou, A.; De Marco, C.; Hu, C.; de Mello, A.J.; Falcaro, P.; et al. MOFBOTS: Metal-Organic-Framework-Based Biomedical Microrobots. Adv. Mater. 2019, 31, 1901592. [Google Scholar] [CrossRef]
- Rapoport, N. Physical stimuli-responsive polymeric micelles for anti-cancer drug delivery. Prog. Polym. Sci. 2007, 32, 962–990. [Google Scholar] [CrossRef]
- Song, Z.; Wu, Y.; Cao, Q.; Wang, H.; Wang, X.; Han, H. pH-Responsive, Light-Triggered on-Demand Antibiotic Release from Functional Metal–Organic Framework for Bacterial Infection Combination Therapy. Adv. Funct. Mater. 2018, 28, 1800011. [Google Scholar] [CrossRef]
- Stefaniak, K.R.; Epley, C.C.; Novak, J.J.; McAndrew, M.L.; Cornell, H.D.; Zhu, J.; McDaniel, D.K.; Davis, J.L.; Allen, I.C.; Morris, A.J.; et al. Photo-triggered release of 5-fluorouracil from a MOF drug delivery vehicle. Chem. Commun. 2018, 54, 7617–7620. [Google Scholar] [CrossRef]
- Zeng, J.-Y.; Zhang, M.-K.; Peng, M.-Y.; Gong, D.; Zhang, X.-Z. Porphyrinic Metal–Organic Frameworks Coated Gold Nanorods as a Versatile Nanoplatform for Combined Photodynamic/Photothermal/Chemotherapy of Tumor. Adv. Funct. Mater. 2018, 28, 1705451. [Google Scholar] [CrossRef]
- Tan, L.L.; Li, H.; Zhou, Y.; Zhang, Y.; Feng, X.; Wang, B.; Yang, Y.W. Zn(2+)-Triggered Drug Release from Biocompatible Zirconium MOFs Equipped with Supramolecular Gates. Small 2015, 11, 3807–3813. [Google Scholar] [CrossRef]
- Tan, L.-L.; Song, N.; Zhang, S.X.-A.; Li, H.; Wang, B.; Yang, Y.-W. Ca2+, pH and thermo triple-responsive mechanized Zr-based MOFs for on-command drug release in bone diseases. J. Mater. Chem. B 2015, 4, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; He, L.; Bian, X.W.; Tian, G. Metal-organic frameworks-based nanozymes for combined cancer therapy. Nano Today 2020, 35, 100920. [Google Scholar] [CrossRef]
- Abánades Lázaro, I.; Forgan, R.S. Application of zirconium MOFs in drug delivery and biomedicine. Coord. Chem. Rev. 2019, 380, 230–259. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.J.; Ji, X.; He, T.; Xie, L.H.; Zhang, Y.Z.; Lv, H.; Ding, C.; Li, J.R. A Green-Emission Metal-Organic Framework-Based Nanoprobe for Imaging Dual Tumor Biomarkers in Living Cells. ACS Appl. Mater. Interfaces 2020, 12, 35375–35384. [Google Scholar] [CrossRef]
- Sheta, S.M.; El-Sheikh, S.M.; Abd-Elzaher, M.M.; Salem, S.R.; Moussa, H.A.; Mohamed, R.M.; Mkhalid, I.A. A novel biosensor for early diagnosis of liver cancer cases using smart nano-magnetic metal–organic framework. Appl. Organomet. Chem. 2019, 33, e5249. [Google Scholar] [CrossRef]
- Kundu, T.; Mitra, S.; Patra, P.; Goswami, A.; Díazdíaz, D.; Banerjee, R. Mechanical downsizing of a gadolinium(III)-based metal-organic framework for anticancer drug delivery. Chem.-A Eur. J. 2014, 20, 10514–10518. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhong, Y.; Wang, X.; Zhuang, C.; Chen, J.; Liu, D.; Xiao, W.; Pan, Y.; Huang, J.; Liu, J. A porous Cu(II)-based metal-organic framework carrier for pH-controlled anticancer drug delivery. Inorg. Chem. Commun. 2020, 111, 107675. [Google Scholar] [CrossRef]
- Zhao, H.X.; Zou, Q.; Sun, S.K.; Yu, C.; Zhang, X.; Li, R.J.; Fu, Y.Y. Theranostic metal-organic framework core-shell composites for magnetic resonance imaging and drug delivery. Chem. Sci. 2016, 7, 5294–5301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, R.; Wang, T.; Zhang, L.; Li, L.; Wang, C. A combination of tri-modal cancer imaging and in vivo drug delivery by metal-organic framework based composite nanoparticles. Biomater. Sci. 2015, 3, 1270–1278. [Google Scholar] [CrossRef]
- Gao, X.; Zhai, M.; Guan, W.; Liu, J.; Liu, Z.; Damirin, A. Controllable synthesis of a smart multifunctional nanoscale metal-organic framework for magnetic resonance/optical imaging and targeted drug delivery. ACS Appl. Mater. Interfaces 2017, 9, 3455–3462. [Google Scholar] [CrossRef]
- Wang, L.; Hou, C.; Yu, H.; Zhang, Q.; Li, Y.; Wang, H. Metal–Organic Framework-Derived Nickel/Cobalt-Based Nanohybrids for Sensing Non-Enzymatic Glucose. ChemElectroChem 2020, 7, 4446–4452. [Google Scholar] [CrossRef]
- Chang, X.; Li, X.; Qiao, X.; Li, K.; Xiong, Y.; Li, X.; Guo, T.; Zhu, L.; Xue, Q. Metal-organic frameworks derived ZnO@MoS2nanosheets core/shell heterojunctions for ppb-level acetone detection: Ultra-fast response and recovery. Sens. Actuators B Chem. 2020, 304, 127430. [Google Scholar] [CrossRef]
- Wang, X.F.; Ma, W.; Jiang, F.; Cao, E.S.; Sun, K.M.; Cheng, L.; Song, X.Z. Prussian Blue analogue derived porous NiFe2O4 nanocubes for low-concentration acetone sensing at low working temperature. Chem. Eng. J. 2018, 338, 504–512. [Google Scholar] [CrossRef]
- Chen, E.X.; Yang, H.; Zhang, J. Zeolitic imidazolate framework as formaldehyde gas sensor. Inorg. Chem. 2014, 53, 5411–5413. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.; Möslein, A.F.; Tan, J.C. Facile and Fast Transformation of Nonluminescent to Highly Luminescent Metal-Organic Frameworks: Acetone Sensing for Diabetes Diagnosis and Lead Capture from Polluted Water. ACS Appl. Mater. Interfaces 2021, 13, 7801–7811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, N.; Cao, P.; Lin, M.; Xu, L.; Ma, H. Electrochemical non-enzymatic glucose sensor using ionic liquid incorporated cobalt-based metal-organic framework. Microchem. J. 2020, 159, 105343. [Google Scholar] [CrossRef]
- Wei, X.; Guo, J.; Lian, H.; Sun, X.; Liu, B. Cobalt metal-organic framework modified carbon cloth/paper hybrid electrochemical button-sensor for nonenzymatic glucose diagnostics. Sens. Actuators B Chem. 2020, 329, 129205. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; Modica, J.A.; Drout, R.J.; Farha, O.K. Acid-Resistant Mesoporous Metal-Organic Framework toward Oral Insulin Delivery: Protein Encapsulation, Protection, and Release. J. Am. Chem. Soc. 2018, 140, 5678–5681. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, L.; Cao, Y.; Yu, S.; He, C.; Chen, X. A Nanocomposite Vehicle Based on Metal-Organic Framework Nanoparticle Incorporated Biodegradable Microspheres for Enhanced Oral Insulin Delivery. ACS Appl. Mater. Interfaces 2020, 12, 22581–22592. [Google Scholar] [CrossRef]
- Zhang, C.; Hong, S.; Liu, M.D.; Yu, W.Y.; Zhang, M.K.; Zhang, L.; Zeng, X.; Zhang, X.Z. pH-sensitive MOF integrated with glucose oxidase for glucose-responsive insulin delivery. J. Control. Release 2020, 320, 159–167. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, S.; Yi, J.; Zhang, H.F.; Ameer, G.A. A Cooperative Copper Metal–Organic Framework-Hydrogel System Improves Wound Healing in Diabetes. Adv. Funct. Mater. 2017, 27, 1604872. [Google Scholar] [CrossRef]
- Xiao, J.; Zhu, Y.; Huddleston, S.; Li, P.; Xiao, B.; Farha, O.K.; Ameer, G.A. Copper Metal-Organic Framework Nanoparticles Stabilized with Folic Acid Improve Wound Healing in Diabetes. ACS Nano 2018, 12, 1023–1032. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Tang, Y.; Shen, H.; Li, J.; Yi, Z.; Ke, Q.; Xu, H. Copper-Based Metal-Organic Framework as a Controllable Nitric Oxide-Releasing Vehicle for Enhanced Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 18319–18331. [Google Scholar] [CrossRef]
- Li, J.; Lv, F.; Li, J.; Li, Y.; Gao, J.; Luo, J.; Xue, F.; Ke, Q.; Xu, H. Cobalt-based metal–organic framework as a dual cooperative controllable release system for accelerating diabetic wound healing. Nano Res. 2020, 13, 2268–2279. [Google Scholar] [CrossRef]
- Xu, B.; Wang, H.; Wang, W.; Gao, L.; Li, S.; Pan, X.; Wang, H.; Yang, H.; Meng, X.; Wu, Q.; et al. A Single-Atom Nanozyme for Wound Disinfection Applications. Angew. Chem.-Int. Ed. 2019, 58, 4911–4916. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A. Neurochemical Aspects of Neurological Disorders. In Trace Amines and Neurological Disorders: Potential Mechanisms and Risk Factors; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 237–256. ISBN 9780128036167. [Google Scholar]
- Chen, H.L.; Li, R.T.; Wu, K.Y.; Hu, P.P.; Zhang, Z.; Huang, N.H.; Zhang, W.H.; Chen, J.X. Experimental and theoretical validations of a one-pot sequential sensing of Hg2+ and biothiols by a 3D Cu-based zwitterionic metal−organic framework. Talanta 2020, 210, 120596. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, Y.; Yang, Y.; Li, B.; Cui, Y.; Qian, G. Post-modified metal-organic framework as a turn-on fluorescent probe for potential diagnosis of neurological diseases. Microporous Mesoporous Mater. 2019, 288, 109610. [Google Scholar] [CrossRef]
- Brambilla, D.; Le Droumaguet, B.; Nicolas, J.; Hashemi, S.H.; Wu, L.P.; Moghimi, S.M.; Couvreur, P.; Andrieux, K. Nanotechnologies for Alzheimer’s disease: Diagnosis, therapy, and safety issues. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 521–540. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zhang, J.; Yu, E.; Cui, Y. Recent progress in metal–organic frameworks for precaution and diagnosis of Alzheimer’s disease. Polyhedron 2018, 151, 554–567. [Google Scholar] [CrossRef]
- Zhang, X.N.; Liu, L.; Han, Z.B.; Gao, M.L.; Yuan, D.Q. A dual-functional Cd(II)-organic-framework demonstrating selective sensing of Zn2+ and Fe3+ ions exclusively and size-selective catalysis towards cyanosilylation. RSC Adv. 2015, 5, 10119–10124. [Google Scholar] [CrossRef]
- Wang, X.; Qin, T.; Bao, S.S.; Zhang, Y.C.; Shen, X.; Zheng, L.M.; Zhu, D. Facile synthesis of a water stable 3D Eu-MOF showing high proton conductivity and its application as a sensitive luminescent sensor for Cu2+ ions. J. Mater. Chem. A 2016, 4, 16484–16489. [Google Scholar] [CrossRef]
- Zhai, B.; Li, Z.Y.; Wu, Z.L.; Cui, J.Z. A novel europium metal-organic framework as luminescent probe for detecting Al3 +. Inorg. Chem. Commun. 2016, 71, 23–26. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Q.; Guo, C.; Sun, Y.; Xie, L.H.; Li, J.R. Stable Zr(IV)-Based Metal-Organic Frameworks with Predesigned Functionalized Ligands for Highly Selective Detection of Fe(III) Ions in Water. ACS Appl. Mater. Interfaces 2017, 9, 10286–10295. [Google Scholar] [CrossRef]
- Zhao, J.; Yin, F.; Ji, L.; Wang, C.; Shi, C.; Liu, X.; Yang, H.; Wang, X.; Kong, L. Development of a Tau-Targeted Drug Delivery System Using a Multifunctional Nanoscale Metal-Organic Framework for Alzheimer’s Disease Therapy. ACS Appl. Mater. Interfaces 2020, 12, 44447–44458. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fan, Y.; Tan, Y.; Zhao, X.; Zhang, Y.; Cheng, C.; Yang, M. Porphyrinic Metal-Organic Framework PCN-224 Nanoparticles for Near-Infrared-Induced Attenuation of Aggregation and Neurotoxicity of Alzheimer’s Amyloid-β Peptide. ACS Appl. Mater. Interfaces 2018, 10, 36615–36621. [Google Scholar] [CrossRef]
- He, J.; Li, C.; Ye, J.; Qiao, Y.; Gu, L. Self-speculation of clinical features based on knowledge distillation for accurate ocular disease classification. Biomed. Signal Process. Control 2021, 67, 102491. [Google Scholar] [CrossRef]
- Eldeeb, A.E.; Salah, S.; Ghorab, M. Formulation and evaluation of cubosomes drug delivery system for treatment of glaucoma: Ex-vivo permeation and in-vivo pharmacodynamic study. J. Drug Deliv. Sci. Technol. 2019, 52, 236–247. [Google Scholar] [CrossRef]
- Gandara-Loe, J.; Ortuno-Lizarán, I.; Fernández-Sanchez, L.; Alió, J.L.; Cuenca, N.; Vega-Estrada, A.; Silvestre-Albero, J. Metal-Organic Frameworks as Drug Delivery Platforms for Ocular Therapeutics. ACS Appl. Mater. Interfaces 2019, 11, 1924–1931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.N.; Park, C.G.; Huh, B.K.; Lee, S.H.; Min, C.H.; Lee, Y.Y.; Kim, Y.K.; Park, K.H.; Choy, Y. Bin Metal-organic frameworks, NH2-MIL-88(Fe), as carriers for ophthalmic delivery of brimonidine. Acta Biomater. 2018, 79, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Gandara-Loe, J.; Souza, B.E.; Missyul, A.; Giraldo, G.; Tan, J.C.; Silvestre-Albero, J. MOF-Based Polymeric Nanocomposite Films as Potential Materials for Drug Delivery Devices in Ocular Therapeutics. ACS Appl. Mater. Interfaces 2020, 12, 30189–30197. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, W.; Yuan, B.; Yin, X.; Li, Y.; Li, Z.; Cui, J.; Yuan, X.; Li, Y. The application of methylprednisolone nanoscale zirconium-porphyrin metal-organic framework (MPS-NPMOF) in the treatment of photoreceptor degeneration. Int. J. Nanomed. 2019, 14, 9763–9776. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Ge, A.; Zhu, H.; Yang, P. A water-stable Zn(II)-based metal-organic framework for selective detection of Fe3+ ion and treatment effect on children asthma by reducing apoptosis of eosinophil. Inorg. Chim. Acta 2020, 506, 119526. [Google Scholar] [CrossRef]
- Zhu, Z.; Natarajan, V.; Wang, W.N. The role of Fe3+ ions in fluorescence detection of H2S by a bimetallic metal-organic framework. J. Solid State Chem. 2020, 288, 121434. [Google Scholar] [CrossRef]
- Li, H.; Zhu, J.; Wang, C.; Qin, W.; Hu, X.; Tong, J.; Yu, L.; Zhang, G.; Ren, X.; Li, Z.; et al. Paeonol loaded cyclodextrin metal-organic framework particles for treatment of acute lung injury via inhalation. Int. J. Pharm. 2020, 587, 119649. [Google Scholar] [CrossRef] [PubMed]
- Strzempek, W.; Menaszek, E.; Gil, B. Fe-MIL-100 as drug delivery system for asthma and chronic obstructive pulmonary disease treatment and diagnosis. Microporous Mesoporous Mater. 2019, 280, 264–270. [Google Scholar] [CrossRef]
- Mao, D.; Hu, F.; Ji, S.; Wu, W.; Ding, D.; Kong, D.; Liu, B. Metal–Organic-Framework-Assisted In Vivo Bacterial Metabolic Labeling and Precise Antibacterial Therapy. Adv. Mater. 2018, 30, 1706831. [Google Scholar] [CrossRef]
- Rahmati, Z.; Abdi, J.; Vossoughi, M.; Alemzadeh, I. Ag-doped magnetic metal organic framework as a novel nanostructured material for highly efficient antibacterial activity. Environ. Res. 2020, 188, 109555. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, X.; He, C.; Huang, J.; Yin, S.; Zhou, M.; Ma, L.; Zhao, W.; Qiu, L.; Cheng, C.; et al. Metal-Organic Framework/Ag-Based Hybrid Nanoagents for Rapid and Synergistic Bacterial Eradication. ACS Appl. Mater. Interfaces 2020, 12, 13698–13708. [Google Scholar] [CrossRef]
- Wyszogrodzka, G.; Dorożyński, P.; Gil, B.; Roth, W.J.; Strzempek, M.; Marszałek, B.; Węglarz, W.P.; Menaszek, E.; Strzempek, W.; Kulinowski, P. Iron-Based Metal-Organic Frameworks as a Theranostic Carrier for Local Tuberculosis Therapy. Pharm. Res. 2018, 35, 144. [Google Scholar] [CrossRef] [Green Version]
- Esfahanian, M.; Ghasemzadeh, M.A.; Razavian, S.M.H. Synthesis, identification and application of the novel metal-organic framework Fe3O4@PAA@ZIF-8 for the drug delivery of ciprofloxacin and investigation of antibacterial activity. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2024–2030. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wu, S. Porous Iron-Carboxylate Metal-Organic Framework: A Novel Bioplatform with Sustained Antibacterial Efficacy and Nontoxicity. ACS Appl. Mater. Interfaces 2017, 9, 19248–19257. [Google Scholar] [CrossRef]
- Gwon, K.; Han, I.; Lee, S.; Kim, Y.; Lee, D.N. Novel Metal-Organic Framework-Based Photocrosslinked Hydrogel System for Efficient Antibacterial Applications. ACS Appl. Mater. Interfaces 2020, 12, 20234–20242. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, S.; Liu, H.; Chen, H.; Zhou, J.; Chen, Z.; Zhou, X.; Xie, Z.; Kuang, Q.; Zheng, L. Biomimetic Metal-Organic Framework Composite-Mediated Cascade Catalysis for Synergistic Bacteria Killing. ACS Appl. Mater. Interfaces 2020, 12, 36996–37005. [Google Scholar] [CrossRef] [PubMed]
- Firouzjaei, M.D.; Shamsabadi, A.A.; Sharifian Gh, M.; Rahimpour, A.; Soroush, M. A Novel Nanocomposite with Superior Antibacterial Activity: A Silver-Based Metal Organic Framework Embellished with Graphene Oxide. Adv. Mater. Interfaces 2018, 5, 1701365. [Google Scholar] [CrossRef]
- Figueira, F.; Barbosa, J.S.; Mendes, R.F.; Braga, S.S.; Paz, F.A.A. Virus meet metal-organic frameworks: A nanoporous solution to a world-sized problem? Mater. Today 2021, 43, 84–98. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; He, Q.; Yan, J.; Xiong, H.; Wen, N.; Cai, S.; Peng, D.; Liu, Y.; Liu, Z. Metal-organic frameworks for virus detection. Biosens. Bioelectron. 2020, 169, 112604. [Google Scholar] [CrossRef]
- Qiu, G.H.; Weng, Z.H.; Hu, P.P.; Duan, W.J.; Xie, B.P.; Sun, B.; Tang, X.Y.; Chen, J.X. Synchronous detection of ebolavirus conserved RNA sequences and ebolavirus-encoded miRNA-like fragment based on a zwitterionic copper (II) metal–organic framework. Talanta 2018, 180, 396–402. [Google Scholar] [CrossRef]
- Qin, L.; Lin, L.X.; Fang, Z.P.; Yang, S.P.; Qiu, G.H.; Chen, J.X.; Chen, W.H. A water-stable metal-organic framework of a zwitterionic carboxylate with dysprosium: A sensing platform for Ebolavirus RNA sequences. Chem. Commun. 2016, 52, 132–135. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Liu, W.S.; Chen, J.S.; Niu, H.L.; Mao, C.J.; Jin, B.K. Metal-organic gel and metal-organic framework based switchable electrochemiluminescence RNA sensing platform for Zika virus. Sens. Actuators B Chem. 2020, 321, 128456. [Google Scholar] [CrossRef]
- Xie, B.P.; Qiu, G.H.; Hu, P.P.; Liang, Z.; Liang, Y.M.; Sun, B.; Bai, L.P.; Jiang, Z.H.; Chen, J.X. Simultaneous detection of Dengue and Zika virus RNA sequences with a three-dimensional Cu-based zwitterionic metal–organic framework, comparison of single and synchronous fluorescence analysis. Sens. Actuators B Chem. 2018, 254, 1133–1140. [Google Scholar] [CrossRef]
- Wang, L.; Liang, K.; Feng, W.; Chen, C.; Gong, H.; Cai, C. Molecularly imprinted polymers based on magnetically fluorescent metal–organic frameworks for the selective detection of hepatitis A virus. Microchem. J. 2021, 164, 106047. [Google Scholar] [CrossRef]
- Yang, J.; Feng, W.; Liang, K.; Chen, C.; Cai, C. A novel fluorescence molecularly imprinted sensor for Japanese encephalitis virus detection based on metal organic frameworks and passivation-enhanced selectivity. Talanta 2020, 212, 120744. [Google Scholar] [CrossRef]
- Marcos-Almaraz, M.T.; Gref, R.; Agostoni, V.; Kreuz, C.; Clayette, P.; Serre, C.; Couvreur, P.; Horcajada, P. Towards improved HIV-microbicide activity through the co-encapsulation of NRTI drugs in biocompatible metal organic framework nanocarriers. J. Mater. Chem. B 2017, 5, 8563–8569. [Google Scholar] [CrossRef] [PubMed]
- Fakharzadeh, S.; Argani, H.; Torbati, P.M.; Dadashzadeh, S.; Kalanaky, S.; Nazaran, M.H.; Basiri, A. DIBc nano metal-organic framework improves biochemical and pathological parameters of experimental chronic kidney disease. J. Trace Elem. Med. Biol. 2020, 61, 126547. [Google Scholar] [CrossRef]
- El-Shafey, A.A.M.; Hegab, M.H.A.; Seliem, M.M.E.; Barakat, A.M.A.; Mostafa, N.E.; Abdel-Maksoud, H.A.; Abdelhameed, R.M. Curcumin@metal organic frameworks nano-composite for treatment of chronic toxoplasmosis. J. Mater. Sci. Mater. Med. 2020, 31, 90. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Liu, J.; Qin, Y.; Wang, H.; Cao, B.; Lu, L.; Yu, X. Metal-Organic Framework Encapsulating Hemoglobin as a High-Stable and Long-Circulating Oxygen Carriers to Treat Hemorrhagic Shock. ACS Appl. Mater. Interfaces 2019, 11, 35604–35612. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal-organic frameworks in biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef]








| Nanocarriers | Structures | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Liposomes (organic) |  | Biocompatibility, biodegradability, lower toxicity, prevention of drug degradation, reduction in side effects when encapsulating therapeutic agents, targeted drug delivery to diseased tissues, capable of delivering both hydrophilic and hydrophobic drugs, cost-effective formulations of expensive drugs | Instability, low encapsulation efficiency, insufficient drug loading, poor controlled drug release, shorter circulation times in the blood, poor storage stability, weak chemical and physical protection of sensitive drugs | [30,31,32,33,34,35,36,37,38] |
| Polymeric Micelles (PMs) (organic) |  | Biostability, high drug loading capacity, easy to functionalize their surface, long circulation times in blood, reduction in side effects, targeted and controlled drug release | Immature drug release, prone to aggregation and opsonization in the bloodstream | [24,39,40,41] |
| Carbon Nanotubes (CNTs) (inorganic) |  | Dynamic strength, unique physiochemical properties, high drug entanglement, intrinsic stability, good biocompatibility, structural flexibility, suitable surface functionalization, low cytotoxicity | Potential asbestos-like effects, low drug delivery capacity within the CNTs | [3,6,11,27] |
| Quantum Dots (QDs) (inorganic) |  | Distinctive electronic properties, luminescence features, high light stability, continuous absorption, narrow emission bandwidth, versatile surface chemistry | Derivatization and ligands required | [6,42,43] |
| Biomedical Application | Technique | Description | Reference |
|---|---|---|---|
| Drug Delivery | Encapsulation of therapeutic cargoes | Includes:
| [127,128] |
| Conjugations of therapeutic agents to the linkers | The attachment of therapeutic agents to ligands via orthogonal conjugation. An example would be the incorporation of amino groups into the framework by doping the terephthalic acid ligand with 2-aminoterephthalic acid during the growth of Fe-MIL-101 MOFs. | [129] | |
| Therapeutic agents as linkers | Direct incorporation of therapeutics as building blocks for MOF synthesis such as the synthesis of MOFs with porphyrin derivative-based linkers for photodynamic therapy (PDT). An example would be the synthesis of Hf–porphyrin nanoscale DBP–UiO MOF. | [130] | |
| Bioimaging | Magnetic Resonance Imaging (MRI) | A diagnostic method dependent on the nuclear magnetic resonance of particles in the body that produces computerized images by analyzing the absorption and transmission of high-frequency radio waves. Mn, Fe, and Gd-based MOFs are some examples of potential candidates for this application. | [47,131,132] |
| X-ray Computed Tomography Imaging (CT) | A 3-D visualization of internal structures based on the mitigation of X-rays that produces a sequence of tomographic images at various orientations. Photoactive (UiO-PDT) MOF is an example of a CT contrast agent | [133] | |
| Optical Imaging (OI) | Light illumination is used to achieve real-time visualization with minimally invasive operations. An example is the incorporation of indocyanine green (ICG) into MIL-100(Fe) with a high loading capacity of 40 wt%, which is coated with a layer of hyaluronic acid (HA) for tumor-targeting. | [134] | |
| Positron Emission Tomography (PET) | High-resolution 3D images of metabolic processes in the body are given by recording the positrons emission from radioactive materials piling up at the target organs or tissues. Zr-based MOFs were used for PET imaging. | [135] | |
| Biosensing | Nucleic acid sensing | Nucleic acid levels facilitate disease diagnosis and observe biological systems since DNAs and RNAs are crucial for physiological control. The incorporation of the triplex-forming oligonucleotide with H2dtoaCu MOFs to detect HIV DNA sequences is one example of this method. | [136] |
| Intracellular molecules sensing | Many diseases can be reflected by the presence of small biomolecules such as glucose and metal ions in the tissue. R-UiO based bio-MOF was used as a phosphorescence/fluorescence dual-emissive platform for intracellular oxygen ratio measurement. | [137] | |
| Intracellular pH sensing | The reflection of the alternations of physiological environments. F-UiO MOFs were developed for real-time intracellular pH sensing via conjugating fluorescein isothiocyanate with the Zr-based MOFs | [138] | |
| Intracellular temperature sensing | The detection of the temperature difference between normal and diseased cells. Thermosensitive near-infrared LnMOF is an example. | [139] | |
| Biocatalysis | Biomimetic catalysis | Certain MOFs have very effective catalysis as well as very low toxicity features. This makes them appropriate candidates for disease diagnosis and immunoassay. Nanometric MIL-100 implemented the intrinsic peroxidase-like catalytic activity for ascorbic acid colorimetric sensing. | [140] |
| Neurological Disorders | Examples |
|---|---|
| Neurotraumatic diseases | Stroke, spinal cord injury, and traumatic brain injury |
| Neurodegenerative diseases | Alzheimer’s, Parkinsons, and Huntingtons |
| Neuropsychiatric diseases | Autism, depression, and hyperactivity |
| Metal Ion | MOF | Remarks | Reference |
|---|---|---|---|
| Zn2+ | Cd2(L1)(DMF)2(H2O)2 | Zinc ions were selectively fluorescent detected over mixed metal ions in a methanol solution. | [206] |
| Cu2+ | [Me2NH2][Eu(ox)2(H2O)]·3H2O | A 3D Eu-MOF was decomposed upon the exchange of copper ions with a cationic guest molecule, leading to luminescent quenching. | [207] |
| Al3+ | Eu(L4)(OAc)(DMA) | The attachment of aluminum ions on the probe’s surface reduces the energy transfer between Eu3+ and the ligand, resulting in luminescent quenching. | [208] |
| Fe3+ | BUT-14 BUT-15 | BUT-15 showed a better sensing ability as its pyridine N donors donate their long-pair electrons to Fe3+ ions. | [209] |
| Ocular Disease | Description | MOF Nanocarrier | Drug | Loading Capacity | Remarks | Reference |
|---|---|---|---|---|---|---|
| Glaucoma |
| UiO-67 MIL-100 (Fe) | Brimonidine | 50–60 wt% | Cytotoxicity assays showed the high biocompatibility of the MOFs. | [214] |
| NH2-MIL-88(Fe) | 121.3 μg mg−1 | In vivo studies showed that the nanocarriers stayed on the preocular surface for a long period (4 h), resulting in an increase in drug bioavailability. | [215] | |||
| Zr-based UiO-67 and polyurethane MOF (UiO-67@ PU) | 58.4 mg g−1 | The MOF-based polymer nanocomposite showed a prolonged drug release (14 days). | [216] | |||
| Photoreceptor Degeneration |
| Nanoscale zirconium-porphyrin MOF (NPMOF) | Methylprednisolone (MPS) | 97.3 wt% | NPMOF demonstrated excellent in vivo biocompatibility and low biotoxicity. After one intraocular injection, faster photoreceptor regeneration of the retina was achieved with better visual function. | [217] |
| MOF Platform | Bacteria | Drug | Remarks | Reference |
|---|---|---|---|---|
| Fe-MIL-101-NH2 | Mycobacterium tuberculosis | Isoniazid | A theranostic agent for drug delivery and imaging properties. The drug dissolution showed continuous drug release inside the L929 fibroblast cells. | [225] |
| MIL-100 (Fe) nanoparticles (NPs) | Bacteria membrane | 3-azido-d-alanine (D-AzAla) | Fast degradation and accumulation after intravenous injection. Selective integration of d-AzAla into the cell walls of bacteria. | [222] |
| MOF-53 (Fe) nanoparticles (NPs) | Staphylococcus aureus | vancomycin (Van) | Efficient drug loading capacity of 20 wt% and high antibacterial efficiency of 99.3%. Excellent stability under acidic conditions. Excellent biocompatibility. | [227] |
| Fe3O4@PAA@ZIF-8 | Escherichia coli Staphylococcus aureus | ciprofloxacin (CIP) | High loading capacity (93%) and drug release (73%). Inhibition of bacterial growth. | [226] |
| Ag-doped magnetic microporous γ-Fe2O3@SiO2@ZIF-8-Ag (FSZ-Ag) | Escherichia coli Staphylococcus aureus | - | Release of 80% of Ag in the solutions, leading to the suppression of bacteria growth. | [223] |
| hydrogel@ Cu-MOF | Escherichia coli Staphylococcus aureus | - | Excellent antibacterial activity at 2 mg mL−1 due to the large surface area to volume ratio and the antibacterial property of copper. | [228] |
| Ag-doped carbonized ZIF nanocomposites(C-Zn/Ag) | Escherichia coli Staphylococcus aureus | - | Fast and safe wound sterilization and can be an alternative to antibiotics. 100% bactericidal ratio for highly concentrated bacteria (107 CFU/mL) within 10 min. | [224] |
| L-arginine and glucose oxidase encapsulated Cu-MOFs (L-Arg/GOx@CuBDC) | Escherichia coli Staphylococcus aureus | - | Coencapsulation of glucose oxidase (GOx) and l-arginine (l-Arg). High antibacterial efficiency of ≥97% at very low doses. | [229] |
| Silver-based metal−organic framework embellished with graphene-oxide (GO-Ag-MOF) | Escherichia coli Bacillus subtilis | - | Outstanding antibacterial activity Elimination of 95% of live bacteria. | [230] |
| Disease | Description | MOF platform | Remarks | Reference |
|---|---|---|---|---|
| Chronic kidney disease (CKD) | It is defined by the gradual loss of kidney function over time. | DIBc NMOF | Significant improvement and recovery of glomerular basement membrane thickening and wrinkling, mesangial matrix expansion, meningeal hypercellularity, and intra-cytoplasmic hyaline droplets after 8 weeks of treating male Wistar rats with DIBc. | [240] |
| Chronic Toxoplasmosis | It is caused by infection with the Toxoplasma gondii parasite | Curcumin@Fe-MOF and UiO-66-NH2 | Treatment of infected rats with these nanocomposites resulted in a significant decrease in the number of brain cysts (parasite load). | [241] |
| Hemorrhagic Shock | It occurs due to large amounts of blood loss which leads to reduced cardiac output and tissue perfusion | ZIF-8 encapsulating free hemoglobin (ZIF-8@Hb) | Better biocompatibility, less protein adsorption, and macrophage uptake. Extension of blood circulation and reduction of nonspecific distribution in normal organs. Significant extension of survival time was observed in treated mice. | [242] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Sharabati, M.; Sabouni, R.; Husseini, G.A. Biomedical Applications of Metal−Organic Frameworks for Disease Diagnosis and Drug Delivery: A Review. Nanomaterials 2022, 12, 277. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12020277
Al Sharabati M, Sabouni R, Husseini GA. Biomedical Applications of Metal−Organic Frameworks for Disease Diagnosis and Drug Delivery: A Review. Nanomaterials. 2022; 12(2):277. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12020277
Chicago/Turabian StyleAl Sharabati, Miral, Rana Sabouni, and Ghaleb A. Husseini. 2022. "Biomedical Applications of Metal−Organic Frameworks for Disease Diagnosis and Drug Delivery: A Review" Nanomaterials 12, no. 2: 277. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12020277