Effect of Multiply Twinned Ag(0) Nanoparticles on Photocatalytic Properties of TiO2 Nanosheets and TiO2 Nanostructured Thin Films
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Sample Preparation
2.2.1. Ag_TiO2 Nanosheets
2.2.2. Ag_TiO2 Nanostructured Thin Films (NSTFs)
2.3. Characterization Methods
2.4. Photocatalytic Degradation of 4-CP
2.5. Photoelectrochemical Measurements
3. Results and Discussions
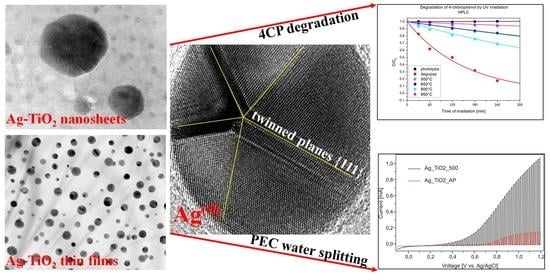
3.1. Microstructure and Surface Characterization of Ag_TiO2 Nanosheets
3.2. Photocatalytic Decomposition of 4-CP and TOC
3.3. Structural Analysis and Surface Characterization of Ag_TiO2_AP and Ag_TiO2_500 NSTFs
3.4. The Key Role of Multiply Twinned Ag(0) NPs on the Photocatalytic Properties of TiO2 Nanosheets and TiO2 NSTFs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Chong, M.; Jin, B.; Chow, C.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Boukherrroub, R.; Ogale, S.B.; Robertson, N. Nanostructured Photocatalysts: From Materials to Applications in Solar Fuels and Environmental Remediation, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-817836-2. [Google Scholar] [CrossRef]
- Potocnik, J. Commission recommendation of 18 October 2011 on the definition of nanomaterial. Off. J. Eur. Communities Legis. 2011, L275, 38–40. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagadevan, S. Semiconductor Nanomaterials, Methods and Applications: A Review. Nanosci. Nanotechnol. 2013, 3, 62–74. [Google Scholar] [CrossRef]
- Kowalska, E.; Remita, H.; Colbeau-Justin, C.; Hupka, J.; Belloni, J. Modification of Titanium Dioxide with Platinum Ions and Clusters, Application in Photocatalysis. J. Phys. Chem. C 2008, 112, 1124–1131. [Google Scholar] [CrossRef]
- Alaoui, O.T.; Herissan, A.; Le Quoc, C.; Zekri, M.; Sorgues, S.; Remita, H.; Colbeau-Justin, C. Elaboration Charge-Carrier Lifetimes and Activity of Pd-TiO2 Obtained by Gamma Radiolysis. J. Photochem. Photobiol. A 2012, 242, 34–43. [Google Scholar] [CrossRef]
- Grabowska, E.; Zaleska, A.; Sorgues, S.; Kunst, M.; Etcheberry, A.; Colbeau-Justin, C.; Remita, H. Modification of Titanium(IV) Dioxide with Small Silver Nanoparticles: Application in Photocatalysis. J. Phys. Chem. C 2013, 117, 1955–1962. [Google Scholar] [CrossRef]
- Štengl, V.; Bakardjieva, S.; Murafa, N. Preparation and photocatalytic activity of rare-earth doped TiO2 nanoparticles. Mater. Chem. Phys. 2009, 114, 217–226. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; Qaiss, A.; Bouhfid, R. Recent progress on Ag/TiO2 photocatalysts: Photocatalytic and bactericidal behaviors. Environ. Sci. Pollut. Res. Int. 2021, 2, 1–29. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, Y.-W. Ag/TiO2 sol prepared by sol-gel methods and its photocatalytic activity. J. Phys. Chem. Solid 2011, 72, 1312–1318. [Google Scholar] [CrossRef]
- Belet, A.; Wolf, C.; Mahy, J.G.; Poelman, D.; Vreuls, C.; Gillard, N.; Lambert, D. Sol-gel syntheses of photocatalysts for the removal of pharmaceutical products in water. Nanomaterials 2019, 9, 126. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Wu, H.S.; Lee, C.Y.; Hung, Y. Synthesis and bactericidal ability of TiO2 and Ag-TiO2 prepared by coprecipitation method. Int. J. Photoenergy 2012, 2012, 640487. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Xia, Y.; Yang, D.; Liu, J.; Zhu, X.; Tang, R. Effects of Hydrothermal Time on Structure and Photocatalytic Property of Titanium Dioxide for Degradation of Rhodamine B and Tetracycline Hydrochloride. Materials 2021, 14, 5674. [Google Scholar] [CrossRef]
- Hariharan, D.; Thangamuniyandi, P.; Christy, A.J.; Vasantharaja, R.; Selvakumar, P.; Sagadeven, S.; Pugazhendhi, A.; Nehru, L.C. Enhanced photocatalysis and anticancer activity of green hydrothermal synthesized Ag@TiO2 nanoparticles. J. Photochem. Photobiol. B Biol. 2020, 202, 111636. [Google Scholar] [CrossRef]
- Sandoval, A.; Aguilar, A.; Louis, C.; Traverse, A.; Zanella, R. Bimetalic Au-Ag/TiO2 catalyst prepared by deposition-precipitation: High activity and stability in CO oxidation. J Catal. 2011, 281, 40–49. [Google Scholar] [CrossRef]
- Chi Chan, S.; Barteau, M.A. Preparation of Highly Uniform Ag/TiO2 and Au/TiO2 Supported Nanoparticle Catalysts by Photodeposition. Langmuir 2005, 21, 5588–5595. [Google Scholar] [CrossRef]
- Li, W.; Lu, K.; Walz, J.Y. Freeze Casting of Porous Materials: Review of Critical Factors in Microstructure Evolution. Int. Mater. Rev. 2012, 57, 37–60. [Google Scholar] [CrossRef]
- Chino, Y.; Dunand, D.C. Directionally freeze-cast titanium foam with aligned, elongated pores. Acta Mater. 2008, 56, 105–113. [Google Scholar] [CrossRef]
- Méndez-Medrano, M.G.; Kowalska, E.; Lehoux, A.; Herissan, A.; Ohtani, B.; Bahena, D.; Briois, V.; Colbeau-Justin, C.; Rodríguez-López, J.L.; Remita, H. Surface Modification of TiO2 with Ag Nanoparticles and CuO Nanoclusters for Application in Photocatalysis. J. Phys. Chem. C 2016, 120, 5143–5154. [Google Scholar] [CrossRef]
- Jiang, B.; Hou, Z.; Tian, C.; Zhou, W.; Zhang, X.; Wu, A.; Tian, G.; Pan, K.; Ren, Z.; Fu, H. A facile and green synthesis route towards twodimensional TiO2@Ag heterojunction structure with enhanced visible-light photocatalytic activity. Cryst. Eng. Comm. 2013, 15, 5821–5827. [Google Scholar] [CrossRef]
- Krenek, T.; Vala, L.; Kovarik, T.; Medlin, R.; Fajgar, R.; Pola, J.; Jandova, V.; Vavrunkova, V.; Pola, M.; Kostejn, M. Novel perspectives of laser ablation in liquids: The formation of a high-pressure orthorhombic FeS phase and absorption of FeS-derived colloids on a porous surface for solar-light photocatalytic wastewater cleaning. Dalton Trans. 2020, 38, 13262–13275. [Google Scholar] [CrossRef]
- Liu, C.H.; Hong, M.H.; Zhou, Y.; Chen, G.X. Synthesis and characterization of Ag deposited TiO2 particles by laser ablation in water. Phys. Scr. 2007, T129, 326. [Google Scholar] [CrossRef]
- Bakardjieva, S.; Subrt, J.; Pulisova, P.; Marikova, M.; Szatmary, L. Photoactivity of Anatase–Rutile TiO2 Nanocrystalline Mixtures Obtained by Heat Treatment of Titanium Peroxide. Gel. Mater. Res. Soc. Symp. Proc. 2011, 10, 1352. [Google Scholar] [CrossRef]
- Šubrt, J.; Pulišová, P.; Boháček, J.; Bezdička, P.; Pližingrová, E.; Volfová, L.; Kupčík, J. Highly photoactive 2D titanium dioxide nanostructures prepared from lyophilized aqueous colloids of peroxo-polytitanic acid. Mat. Res. Bull. 2014, 49, 405–412. [Google Scholar] [CrossRef]
- Plizingrova, E.; Klementova, M.; Bezdicka, P.; Bohacek, J.; Barbierikova, Z.; Dvoranova, D.; Krysa, J.; Subrt, J.; Brezova, V. 2D-Titanium dioxide nanosheets modified with Nd, Ag and Au: Preparation, characterization and photocatalytic activity. Catal. Today 2017, 281, 165–180. [Google Scholar] [CrossRef]
- Bakardjieva, S.; Subrt, J.; Stengl, V.; Dianez, M.J.; Sayagues, M.J. Photoactivity of anatase–rutile TiO2 nanocrystalline mixtures obtained by heat treatment of homogeneously precipitated anatase. Appl. Catal. B: Environ. 2005, 58, 193–202. [Google Scholar] [CrossRef]
- JCPDS PDF-2 Release 2001; ICDD: Newtown Square, PA, USA, 2001.
- ICSD Database; FIZ: Karlsruhe, Germany, 2015.
- Cullity, B.D. Elements of X-ray Diffraction; Addison–Wesley: Reading, MA, USA, 1978; p. 555. [Google Scholar]
- Lábár, J.L. Consistent Indexing of a (Set of) SAED Pattern(s) with the ProcessDiffraction Program. Ultramicroscopy 2005, 103, 237–249. [Google Scholar] [CrossRef]
- Keiderling, U.; Jafta, C. V4: The Small Angle Scattering Instrument (SANS) at BER II. J. Large-Scale Res. Facil. JLSRF 2016, 2, 97. [Google Scholar] [CrossRef]
- Al-Ekabi and Serpone, N. Kinetic studies in heterogeneous photocatalysis. 1. Photocatalytic degradation of chlorinated phenols in aerated aqueous solution over TiO2 supported on a glass matrix. J. Phys. Chem. 1988, 92, 5726–5731. [Google Scholar]
- Gratzel, M.; Serpone, N.; Pelizzetti, E. Photocatalysis, Fundamentals and Applications; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Bakardjieva, S.; Mares, J.; Fajgar, R.; Zenou, V.Y.; Maleckova, M.; Chatzisymeon, E.; Bibova, H.; Jirkovsky, J. The relationship between microstructure and photocatalytic behavior in lanthanum-modified 2DTiO2 nanosheets upon annealing of a freeze-cast precursor. RSC Adv. 2019, 9, 22988. [Google Scholar] [CrossRef] [Green Version]
- Mehta, B.K.; Chhajlani, M.; Shrivastava, B.D. Green synthesis of silver nanoparticles and their characterization by XRD. J. Phys Conf. Ser. 2017, 836, 012050. [Google Scholar] [CrossRef] [Green Version]
- Zenou, V.Y.; Bakardjieva, S. Microstructural analysis of undoped and moderately Sc-doped TiO2 anatase nanoparticles using Scherrer equation and Debye function analysis. Mater. Charact. 2018, 144, 287–296. [Google Scholar] [CrossRef]
- Bakardjieva, S.; Jakubec, I.; Koci, E.; Zhigunov, A.; Chatzisymeon, E.; Davididou, K. Photocatalytic Degradation of Bisphenol A Induced by Dense Nanocavities, Inside Aligned 2D-TiO2 Nanostructures. Catal. Today 2019, 328, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Aadil, M.; Zulfiqar, S.; Agboola, P.O.; Aly Aboud, M.F.; Shakir, I.; Farooq Warsi, M. Fabrication of graphene supported binary nanohybrid with multiple approaches for electrochemical energy storage applications. Synth. Met. 2021, 272, 116645. [Google Scholar] [CrossRef]
- Edelson, L.H.; Glaeser, A.M. Role of Particle Substructure in the Sintering of Monosized Titania. J. Am. Ceram. Soc. 1988, 71, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics. Angew. Chem. Int. Ed. 2009, 48, 60–103. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Chen, X.; Zhao, X.; Gu, L.; Huang, H.; Yan, J.; Xu, C.; Li, G.; Wu, J.; et al. Plasmonic twinned silver nanoparticles with molecular precision. Nat. Commun. 2016, 7, 12809. [Google Scholar] [CrossRef]
- Narayanan, S.; Cheng, G.; Zeng, Z.; Zhu, Y.; Zhu, T. Strain Hardening and Size Effect in Five-fold Twinned Ag Nanowires. Nano Lett. 2015, 15, 4037–4044. [Google Scholar] [CrossRef]
- Mukherji, S.; Bharti, S.; Shukla, G. Synthesis and characterization of size- and shape-controlled silver nanoparticles. Phys. Sci. Rev. 2018, 4, 20170082. [Google Scholar]
- Palmer, D.C. Begbroke; CrystalMaker Software, Ltd.: Oxfordshire, UK, 2014. [Google Scholar]
- Esparza, R.; Rosas, G.; Valenzuela, E.; Gamboa, S.A.; Pal, U.; Pérez, R. Structural analysis and shape-dependent catalytic activity of Au, Pt and Au/Pt nanoparticles. Rev. Mater. 2008, 13, 579–586. [Google Scholar] [CrossRef]
- Rupich, S.M.; Shevchenko, E.V.; Bodnarchuk, M.I.; Lee, B.; Talapin, D.V. Size-Dependent Multiple Twinning in Nanocrystal Superlattices. J. Am. Chem. Soc. 2010, 132, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, G.; Sun, J. Grain Boundary Segregation in Nanocrysturtured Metallic Materials; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Liu, C.-J.; Ge, Q. Interaction of Pt Clusters with the Anatase TiO2(101) Surface: A First-Principles Study. J. Phys. Chem. B 2006, 110, 7463–7472. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Chávez, L.A.; Rivera-Muñoz, E.M.; Velázquez-Castillo, R.R.; Escobar-Alarcón, L.; Esquivel, K. Au-Ag/TiO2 Thin Films Preparation by Laser Ablation and SputteringPlasmas for Its Potential Use as Photoanodes in Electrochemical Advanced Oxidation Processes (EAOP). Catalysts 2021, 11, 1406. [Google Scholar] [CrossRef]
- Malagutti, A.R.; Mourao, H.; Garbin, J.R.; Ribeiro, C. Deposition of TiO2 and Ag:TiO2 thin films by the polymeric precursor method and their application in the photodegradation of textile dyes. Appl. Catal. B Environ. 2009, 90, 205–212. [Google Scholar] [CrossRef]
- Sun, X.; Jiang, K.; Zhang, N.; Guo, S.; Huang, X. Crystalline control of {111} bounded Pt3Cu nanocrystals: Multiply-twinned Pt3Cu icosahedra with enhanced electrocatalytic properties. ACS Nano. 2015, 9, 7634–7640. [Google Scholar] [CrossRef]
- Serpone, N.; Al-Ekabi, H.; Patterson, B.; Pelizzetti, E.; Minero, C.; Pramauro, E.; Fox, M.A.; Draper, B. Kinetic studies in heterogeneous photocatalysis. 2. TiO2 mediated degradation of 4-chlorophenol alone and in a three-component mixture of 4-chlorophenol, 2,4-dichlorophenol, and 2,4,5-trichlorophenol in air-equilibrated aqueous media. Lungmuir 1989, 5, 250. [Google Scholar]
- Al-Sayyed, G.; D’Oliveira, J.C.; Pichat, P. Semiconductor-sensitized photodegradation of 4-chlorophenol in water. J. Photochem. Photobiol A Chem. 1991, 58, 99–114. [Google Scholar] [CrossRef]

















| Sample | Annealing Temperature (°C) | Lattice Parameters (Å) | Anatase by XRD (%) | Rutile by XRD (%) | Anatase Crystallite Size (nm) | Rutile Crystallite Size (nm) | Rate Constants k (s−1) | |
|---|---|---|---|---|---|---|---|---|
| a | c | |||||||
| Ag_TiO_LYO/500 | 500 | 3.7854 | 9.5067 | 100 | - | 35.37 | - | 1.28 × 10−5 |
| Ag_TiO_LYO/650 | 650 | 3.7841 | 9.5103 | 100 | - | 42.68 | - | 1.24 × 10−5 |
| Ag_TiO_LYO/800 | 800 | 3.7848 | 9.5128 | 100 | - | 78.41 | - | 2.53 × 10−5 |
| Ag_TiO_LYO/950 | 950 | 3.7850 | 9.5129 | 32.76 | 66.53 | 238.7 | 251.1 | 3.48 × 10−6 |
| Silver (Ag0) | 950 | 4.0871 | 4.0871 | - | - | - | - | - |
| TiO2_P25 | - | - | - | 83 | 17 | 20.8 | 30.5 | 1.10.10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakardjieva, S.; Mares, J.; Koci, E.; Tolasz, J.; Fajgar, R.; Ryukhtin, V.; Klementova, M.; Michna, Š.; Bibova, H.; Holmestad, R.; et al. Effect of Multiply Twinned Ag(0) Nanoparticles on Photocatalytic Properties of TiO2 Nanosheets and TiO2 Nanostructured Thin Films. Nanomaterials 2022, 12, 750. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12050750
Bakardjieva S, Mares J, Koci E, Tolasz J, Fajgar R, Ryukhtin V, Klementova M, Michna Š, Bibova H, Holmestad R, et al. Effect of Multiply Twinned Ag(0) Nanoparticles on Photocatalytic Properties of TiO2 Nanosheets and TiO2 Nanostructured Thin Films. Nanomaterials. 2022; 12(5):750. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12050750
Chicago/Turabian StyleBakardjieva, Snejana, Jakub Mares, Eva Koci, Jakub Tolasz, Radek Fajgar, Vasyl Ryukhtin, Mariana Klementova, Štefan Michna, Hana Bibova, Randi Holmestad, and et al. 2022. "Effect of Multiply Twinned Ag(0) Nanoparticles on Photocatalytic Properties of TiO2 Nanosheets and TiO2 Nanostructured Thin Films" Nanomaterials 12, no. 5: 750. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12050750