In Situ Growth of W2C/WS2 with Carbon-Nanotube Networks for Lithium-Ion Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Materials
2.2. Synthesis of WCNT
2.3. Material Characterization
2.4. Electrochemical Measurements
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuc, A.; Heine, T. The electronic structure calculations of two-dimensional transition-metal dichalcogenides in the presence of external electric and magnetic fields. Chem. Soc. Rev. 2015, 44, 2603–2614. [Google Scholar] [CrossRef] [PubMed]
- Haque, F.; Daeneke, T.; Kalantar-zadeh, K.; Ou, J.Z. Two-Dimensional Transition Metal Oxide and Chalcogenide-Based Photocatalysts. Nano-Micro Lett. 2017, 10, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Quesne, M.G.; Roldan, A.; de Leeuw, N.H.; Catlow, C.R.A. Bulk and surface properties of metal carbides: Implications for catalysis. Phys. Chem. Chem. Phys. 2018, 20, 6905–6916. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Xinhui, X.; Fan, S.; Jiye, Z.; Jiangping, T.; Jin, F.H. Transition Metal Carbides and Nitrides in Energy Storage and Conversion. Adv. Sci. 2016, 3, 1500286. [Google Scholar]
- Gao, G.; O’Mullane, A.P.; Du, A. 2D MXenes: A New Family of Promising Catalysts for the Hydrogen Evolution Reaction. ACS Catal. 2017, 7, 494–500. [Google Scholar] [CrossRef]
- Pan, H. Ultra-high electrochemical catalytic activity of MXenes. Sci. Rep. 2016, 6, 32531. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.P.; Tuan Nguyen, D.M.; Tran, D.L.; Le, H.K.; Vo, D.-V.N.; Lam, S.S.; Varma, R.S.; Shokouhimehr, M.; Nguyen, C.C.; Le, Q.V. MXenes: Applications in electrocatalytic, photocatalytic hydrogen evolution reaction and CO2 reduction. Mol. Catal. 2020, 486, 110850. [Google Scholar] [CrossRef]
- Chen, Z.; Gong, W.; Cong, S.; Wang, Z.; Song, G.; Pan, T.; Tang, X.; Chen, J.; Lu, W.; Zhao, Z. Eutectoid-structured WC/W2C heterostructures: A new platform for long-term alkaline hydrogen evolution reaction at low overpotentials. Nano Energy 2020, 68, 104335. [Google Scholar] [CrossRef]
- Hussain, S.; Shaikh, S.F.; Vikraman, D.; Mane, R.S.; Joo, O.-S.; Naushad, M.; Jung, J. Sputtering and sulfurization-combined synthesis of a transparent WS2 counter electrode and its application to dye-sensitized solar cells. RSC Adv. 2015, 5, 103567–103572. [Google Scholar] [CrossRef]
- Gowtham, B.; Balasubramani, V.; Ramanathan, S.; Ubaidullah, M.; Shaikh, S.F.; Sreedevi, G. Dielectric relaxation, electrical conductivity measurements, electric modulus and impedance analysis of WO3 nanostructures. J. Alloys Compd. 2021, 888, 161490. [Google Scholar] [CrossRef]
- Al-Tahan, M.A.; Dong, Y.; Shrshr, A.E.; Liu, X.; Zhang, R.; Guan, H.; Kang, X.; Wei, R.; Zhang, J. Enormous-sulfur-content cathode and excellent electrochemical performance of Li-S battery accouched by surface engineering of Ni-doped WS2@rGO nanohybrid as a modified separator. J. Colloid Interface Sci. 2022, 609, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Liu, C.; Chen, T.; Li, W.; Zheng, S.; Pi, Y.; Luo, Y.; Pang, H. MXene-Copper/Cobalt Hybrids via Lewis Acidic Molten Salts Etching for High Performance Symmetric Supercapacitors. Angew. Chem. Int. Ed. 2021, 60, 25318–25322. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Xue, H.; Pang, H.; Xu, Q. Metal–organic frameworks as a platform for clean energy applications. EnergyChem 2020, 2, 100027. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Feng, L.-W.; Gu, C.; Li, G.; Su, N.; Wang, G.; Swick, S.M.; Huang, W.; Guo, X.; et al. Hole (donor) and electron (acceptor) transporting organic semiconductors for bulk-heterojunction solar cells. EnergyChem 2020, 2, 100042. [Google Scholar] [CrossRef]
- Kim, S.; Park, J.; Hwang, J.; Lee, J. Effects of functional supports on efficiency and stability of atomically dispersed noble-metal electrocatalysts. EnergyChem 2021, 3, 100054. [Google Scholar] [CrossRef]
- Zheng, S.; Sun, Y.; Xue, H.; Braunstein, P.; Huang, W.; Pang, H. Dual-ligand and hard-soft-acid-base strategies to optimize metal-organic framework nanocrystals for stable electrochemical cycling performance. Natl. Sci. Rev. 2021. [Google Scholar] [CrossRef]
- Kim, H.; Choi, W.; Yoon, J.; Um, J.H.; Lee, W.; Kim, J.; Cabana, J.; Yoon, W.S. Exploring Anomalous Charge Storage in Anode Materials for Next-Generation Li Rechargeable Batteries. Chem. Rev. 2020, 120, 6934–6976. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Yao, J.; Huang, Y.; Xiao, S. Facile synthesis of porous tubular NiO with considerable pseudocapacitance as high capacity and long life anode for lithium-ion batteries. Ceram. Int. 2018, 44, 2568–2577. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Giang, T.T.; Kim, I.T. Restructuring NiO to LiNiO2: Ultrastable and reversible anodes for lithium-ion batteries. Chem. Eng. J. 2022, 437, 135292. [Google Scholar] [CrossRef]
- Feng, C.; Huang, L.; Guo, Z.; Liu, H. Synthesis of tungsten disulfide (WS2) nanoflakes for lithium-ion battery application. Electrochem. Commun. 2007, 9, 119–122. [Google Scholar] [CrossRef]
- Liu, H.; Su, D.; Wang, G.; Qiao, S.Z. An ordered mesoporous WS2 anode material with superior electrochemical performance for lithium-ion batteries. J. Mater. Chem. 2012, 22, 17437–17440. [Google Scholar] [CrossRef]
- George, C.; Morris, A.J.; Modarres, M.H.; De Volder, M. Structural Evolution of Electrochemically Lithiated MoS2 Nanosheets and the Role of Carbon Additive in Li-Ion Batteries. Chem. Mater. 2016, 28, 7304–7310. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Liu, B.; Zhao, Y.; Chen, W.; Mou, D.; Fu, J.; Wang, Y.; Xin, W.; Zhao, L. Structure design of MoS2@Mo2C on nitrogen-doped carbon for enhanced alkaline hydrogen evolution reaction. J. Mater. Sci. 2020, 55, 16197–16210. [Google Scholar] [CrossRef]
- Liu, J.; Wang, P.; Gao, L.; Wang, X.; Yu, H. In situ sulfuration synthesis of heterostructure MoS2–Mo2C@C for boosting the photocatalytic H2 production activity of TiO2. J. Mater. Chem. C 2022, 10, 3121–3128. [Google Scholar] [CrossRef]
- Ihsan, M.; Wang, H.; Majid, S.R.; Yang, J.; Kennedy, S.J.; Guo, Z.; Liu, H.K. MoO2/Mo2C/C spheres as anode materials for lithium-ion batteries. Carbon 2016, 96, 1200–1207. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wu, X.; Zhang, H.; Zhang, J. Interface Designing over WS2/W2C for Enhanced Hydrogen Evolution Catalysis. ACS Appl. Energy Mater. 2018, 1, 3377–3384. [Google Scholar] [CrossRef]
- Zhao, Z.; Qin, F.; Kasiraju, S.; Xie, L.; Alam, M.K.; Chen, S.; Wang, D.; Ren, Z.; Wang, Z.; Grabow, L.C.; et al. Vertically Aligned MoS2/Mo2C hybrid Nanosheets Grown on Carbon Paper for Efficient Electrocatalytic Hydrogen Evolution. ACS Catal. 2017, 7, 7312–7318. [Google Scholar] [CrossRef]
- Cheng, Y.; Pang, K.; Wu, X.; Zhang, Z.; Xu, X.; Ren, J.; Huang, W.; Song, R. In Situ Hydrothermal Synthesis MoS2/Guar Gum Carbon Nanoflowers as Advanced Electrocatalysts for Electrocatalytic Hydrogen Evolution. ACS Sustain. Chem. Eng. 2018, 6, 8688–8696. [Google Scholar] [CrossRef]
- Faizan, M.; Hussain, S.; Vikraman, D.; Ali, B.; Kim, H.-S.; Jung, J.; Nam, K.-W. MoS2@Mo2C hybrid nanostructures formation as an efficient anode material for lithium-ion batteries. J. Mater. Res. Technol. 2021, 14, 2382–2393. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Choi, K.S.; Kim, S.Y.; Lee, T.H.; Jang, H.W.; Van Le, Q.; Kim, I.T. Strategy for controlling the morphology and work function of W2C/WS2 nanoflowers. J. Alloys Compd. 2020, 829, 154582. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Kim, I.T. W2C/WS2 Alloy Nanoflowers as Anode Materials for Lithium-Ion Storage. Nanomaterials 2020, 10, 1336. [Google Scholar] [CrossRef]
- Sun, D.; Wang, M.; Li, Z.; Fan, G.; Fan, L.-Z.; Zhou, A. Two-dimensional Ti3C2 as anode material for Li-ion batteries. Electrochem. Commun. 2014, 47, 80–83. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Qiu, Z.; Wu, X.; Zhou, P.; Zhou, T.; Zhao, J.; Miao, Z.; Zhou, J.; Zhuo, S. Fe3O4@Ti3C2 MXene hybrids with ultrahigh volumetric capacity as an anode material for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 11189–11197. [Google Scholar] [CrossRef]
- Kong, F.; He, X.; Liu, Q.; Qi, X.; Sun, D.; Zheng, Y.; Wang, R.; Bai, Y. Enhanced reversible Li-ion storage in Si@Ti3C2 MXene nanocomposite. Electrochem. Commun. 2018, 97, 16–21. [Google Scholar] [CrossRef]
- Zhang, Y. First principles prediction of two-dimensional tungsten carbide (W2C) monolayer and its Li storage capability. Comput. Condens. Matter 2017, 10, 35–38. [Google Scholar] [CrossRef]
- Bai, Y.-L.; Liu, Y.-S.; Ma, C.; Wang, K.-X.; Chen, J.-S. Neuron-Inspired Design of High-Performance Electrode Materials for Sodium-Ion Batteries. ACS Nano 2018, 12, 11503–11510. [Google Scholar] [CrossRef]
- Landi, B.J.; Ganter, M.J.; Cress, C.D.; Di Leo, R.A.; Raffaelle, R.P. Carbon nanotubes for lithium-ion batteries. Energy Environ. Sci. 2009, 2, 638–654. [Google Scholar] [CrossRef]
- Lu, C.; Liu, W.-W.; Li, H.; Tay, B.K. A binder-free CNT network–MoS2 composite as a high performance anode material in lithium ion batteries. Chem. Commun. 2014, 50, 3338–3340. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, X.; Evanko, B.; Sun, X.; Li, X.; Hou, T.; Cai, S.; Zheng, C.; Hu, W.; Stucky, G.D. High-rate FeS2/CNT neural network nanostructure composite anodes for stable, high-capacity sodium-ion batteries. Nano Energy 2018, 46, 117–127. [Google Scholar] [CrossRef]
- Yan, G.; Wu, C.; Tan, H.; Feng, X.; Yan, L.; Zang, H.; Li, Y. N-Carbon coated P-W2C composite as efficient electrocatalyst for hydrogen evolution reactions over the whole pH range. J. Mater. Chem. A 2017, 5, 765–772. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Kim, S.Y.; Lee, T.H.; Jang, H.W.; Le, Q.V.; Kim, I.T. Facile synthesis of W2C@WS2 alloy nanoflowers and their hydrogen generation performance. Appl. Surf. Sci. 2020, 504, 144389. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Sohn, W.; Oh, J.H.; Jang, H.W.; Kim, S.Y. Size-Dependent Properties of Two-Dimensional MoS2 and WS2. J. Phys. Chem. C 2016, 120, 10078–10085. [Google Scholar] [CrossRef]
- Ren, J.; Ren, R.-P.; Lv, Y.-K. WS2-decorated graphene foam@CNTs hybrid anode for enhanced lithium-ion storage. J. Alloys Compd. 2019, 784, 697–703. [Google Scholar] [CrossRef]
- Vaziri, H.S.; Shokuhfar, A.; Afghahi, S.S.S. Synthesis of WS2/CNT hybrid nanoparticles for fabrication of hybrid aluminum matrix nanocomposite. Mater. Res. Express 2020, 7, 025034. [Google Scholar] [CrossRef]
- Zhang, L.-N.; Ma, Y.-Y.; Lang, Z.-L.; Wang, Y.-H.; Khan, S.U.; Yan, G.; Tan, H.-Q.; Zang, H.-Y.; Li, Y.-G. Ultrafine cable-like WC/W2C heterojunction nanowires covered by graphitic carbon towards highly efficient electrocatalytic hydrogen evolution. J. Mater. Chem. A 2018, 6, 15395–15403. [Google Scholar] [CrossRef]
- Chen, Y.; Song, B.; Tang, X.; Lu, L.; Xue, J. Ultrasmall Fe3O4 Nanoparticle/MoS2 Nanosheet Composites with Superior Performances for Lithium-Ion Batteries. Small 2014, 10, 1536–1543. [Google Scholar] [CrossRef]
- Jin, Y.; Li, S.; Kushima, A.; Zheng, X.; Sun, Y.; Xie, J.; Sun, J.; Xue, W.; Zhou, G.; Wu, J.; et al. Self-healing SEI enables full-cell cycling of a silicon-majority anode with a coulombic efficiency exceeding 99.9%. Energy Environ. Sci. 2017, 10, 580–592. [Google Scholar] [CrossRef]
- Stephenson, T.; Li, Z.; Olsen, B.; Mitlin, D. Lithium-ion battery applications of molybdenum disulfide (MoS2) nanocomposites. Energy Environ. Sci. 2014, 7, 209–231. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, X.J.; Yang, X.Q.; Xun, S.D.; Liu, G.; Koech, P.K.; Liu, J.; Lemmon, J.P. Electrochemically Induced High Capacity Displacement Reaction of PEO/MoS2/Graphene Nanocomposites with Lithium. Adv. Funct. Mater. 2011, 21, 2840–2846. [Google Scholar] [CrossRef]
- Ren, J.; Ren, R.-P.; Lv, Y.-K. A flexible 3D graphene@CNT@MoS2 hybrid foam anode for high-performance lithium-ion battery. Chem. Eng. J. 2018, 353, 419–424. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, B.; Seo, D.H.; Han, Z.J.; Wong, J.I.; Ostrikov, K.; Zhang, H.; Yang, H.Y. MoS2-coated vertical graphene nanosheet for high-performance rechargeable lithium-ion batteries and hydrogen production. NPG Asia Mater. 2016, 8, e268. [Google Scholar] [CrossRef] [Green Version]
- Peled, E.; Menkin, S. Review—SEI: Past, Present and Future. J. Electrochem. Soc. 2017, 164, A1703–A1719. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bao, Z.; Cui, Y.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.; Liaw, B.Y.; Liu, P.; Manthiram, A.; et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4, 180–186. [Google Scholar] [CrossRef]
- Bisquert, J.; Garcia-Belmonte, G.; Bueno, P.; Longo, E.; Bulhões, L.O.S. Impedance of constant phase element (CPE)—Blocked diffusion in film electrodes. J. Electroanal. Chem. 1998, 452, 229–234. [Google Scholar] [CrossRef]
- Piao, T.; Park, S.M.; Doh, C.H.; Moon, S.I. Intercalation of Lithium Ions into Graphite Electrodes Studied by AC Impedance Measurements. J. Electrochem. Soc. 1999, 146, 2794–2798. [Google Scholar] [CrossRef] [Green Version]
- Ye, B.; Xu, L.; Wu, W.; Ye, Y.; Yang, Z.; Ai, J.; Qiu, Y.; Gong, Z.; Zhou, Y.; Huang, Q.; et al. Encapsulation of 2D MoS2 nanosheets into 1D carbon nanobelts as anodes with enhanced lithium/sodium storage properties. J. Mater. Chem. C 2022, 10, 3329–3342. [Google Scholar] [CrossRef]
- Rui, X.H.; Ding, N.; Liu, J.; Li, C.; Chen, C.H. Analysis of the chemical diffusion coefficient of lithium ions in Li3V2(PO4)3 cathode material. Electrochim. Acta 2010, 55, 2384–2390. [Google Scholar] [CrossRef]
- Abdelaal, M.M.; Hung, T.-C.; Mohamed, S.G.; Yang, C.-C.; Huang, H.-P.; Hung, T.-F. A Comparative Study of the Influence of Nitrogen Content and Structural Characteristics of NiS/Nitrogen-Doped Carbon Nanocomposites on Capacitive Performances in Alkaline Medium. Nanomaterials 2021, 11, 1867. [Google Scholar] [CrossRef] [PubMed]
- Bhandavat, R.; David, L.; Singh, G. Synthesis of Surface-Functionalized WS2 Nanosheets and Performance as Li-Ion Battery Anodes. J. Phys. Chem. Lett. 2012, 3, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Liu, H.; Wang, S.; Yang, X.; Liu, B.; Chen, H.; Xu, M. Hierarchical architecture of WS2 nanosheets on graphene frameworks with enhanced electrochemical properties for lithium storage and hydrogen evolution. J. Mater. Chem. A 2015, 3, 24128–24138. [Google Scholar] [CrossRef]
- Chen, D.; Ji, G.; Ding, B.; Ma, Y.; Qu, B.; Chen, W.; Lee, J.Y. In situ nitrogenated graphene—Few-layer WS2 composites for fast and reversible Li+ storage. Nanoscale 2013, 5, 7890–7896. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Ding, Z.; Ma, C.; Wu, L.; Liu, J.; Chen, L.; Ivey, D.G.; Wei, W. Hierarchical Nanocomposite of Hollow N-Doped Carbon Spheres Decorated with Ultrathin WS2 Nanosheets for High-Performance Lithium-Ion Battery Anode. ACS Appl. Mater. Interfaces 2016, 8, 18841–18848. [Google Scholar] [CrossRef] [PubMed]
- Debela, T.T.; Lim, Y.R.; Seo, H.W.; Kwon, I.S.; Kwak, I.H.; Park, J.; Cho, W.I.; Kang, H.S. Two-Dimensional WS2@Nitrogen-Doped Graphite for High-Performance Lithium-Ion Batteries: Experiments and Molecular Dynamics Simulations. ACS Appl. Mater. Interfaces 2018, 10, 37928–37936. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, F.; Yuan, H.; Yang, Z.; Qin, Y.; Zheng, X.; Yang, Y. N-Doped Carbon—WS2 Nanosheet Composites for Lithium-Ion Storage. ACS Appl. Nano Mater. 2021, 4, 7781–7787. [Google Scholar] [CrossRef]







| Anode Materials | Current Density (mA g−1) | Initial Discharge Capacity (mAh g−1) | Cycle Number | Specific Capacity (mAh g−1) | References |
|---|---|---|---|---|---|
| WS2 nanoflakes | 47.5 | ~1700 | 20 | ~700 | [22] |
| Oxygen-functionalized WS2 | 50 | ~920 | 20 | ~220 | [62] |
| Hierarchical WS2 on 3D graphene | 100 | ~800 | 100 | ~740 | [63] |
| Mesoporous WS2 | 100 | ~1300 | 100 | ~800 | [23] |
| N-graphene/WS2 | 100 | ~1300 | 100 | ~800 | [64] |
| N-carbon sphere/WS2 | 100 | ~735 | 100 | ~630 | [65] |
| N-graphene/WS2 | 100 | ~950 | 100 | ~960 | [66] |
| N-carbon/WS2 | 100 | ~1000 | 100 | ~640 | [67] |
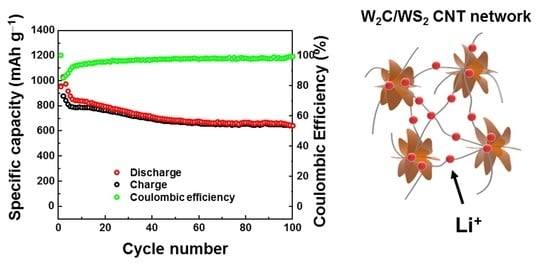
| W2C/WS2/CNTs | 100 | ~1000 | 100 | ~650 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.P.; Kim, I.T. In Situ Growth of W2C/WS2 with Carbon-Nanotube Networks for Lithium-Ion Storage. Nanomaterials 2022, 12, 1003. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12061003
Nguyen TP, Kim IT. In Situ Growth of W2C/WS2 with Carbon-Nanotube Networks for Lithium-Ion Storage. Nanomaterials. 2022; 12(6):1003. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12061003
Chicago/Turabian StyleNguyen, Thang Phan, and Il Tae Kim. 2022. "In Situ Growth of W2C/WS2 with Carbon-Nanotube Networks for Lithium-Ion Storage" Nanomaterials 12, no. 6: 1003. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12061003