The Effect of Ag-Decoration on rGO/Water Nanofluid Thermal Conductivity and Viscosity
Abstract
:1. Introduction
2. Materials and Methods
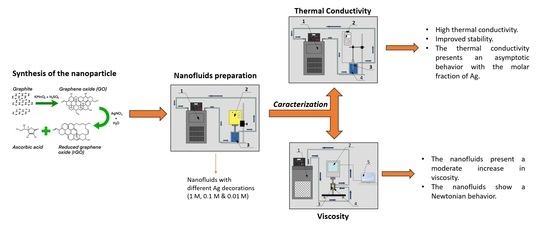
2.1. Synthesis of Ag-Decorated Graphene
2.2. Characterization of the Nanomaterials
2.3. Nanofluid Preparation
2.4. Nanofluid Characterization
3. Results and Analysis
4. Conclusions
- The nanofluid thermal conductivity showed increases concerning the base fluid-the most promising being 28.43 and 26.25% for 0.1 and 1 M of Ag, respectively.
- The thermal conductivity presented asymptotic behavior with the molar fraction of Ag. The experimental data showed that, when increasing the molar fraction from 0.1 to 1 M, the thermal conductivity did not improve significantly.
- Based on the time constant of thermal conductivity transient behavior, the increase in the molar fraction of Ag generated instability in the nanofluids studied. The nanofluid prepared from rGO decorated with 0.1 M of Ag corresponded to an optimum, considering the increase of thermal conductivity and its stability.
- The prepared nanofluids presented a moderate increase (<11%) in viscosity compared to the base fluid.
- The nanofluids showed Newtonian behavior at the studied shear rates (40–106 s), strengthening such behavior when the molar fraction of Ag was increased.
- Due to their improved thermal conductivity and low viscosity, aqueous nanofluids based on rGO/Ag-decorated nanocomposites are a potential alternative to be used as heat-transfer fluids. Therefore, additional studies should be performed.
- For instance, it is recommended to analyze the effect of the rGO/Ag nanocomposite concentration on the thermal properties and rheological behavior of nanofluids as well as implementations of these nanofluids in heat transfer applications.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Choi, S.U.S.; Eastman, J.A. Enhancing Thermal Conductivity of Fluids with Nanoparticles; Argonne National Lab: Argonne, IL, USA, 1995. [Google Scholar]
- Sadeghinezhad, E.; Mehrali, M.; Latibari, S.T.; Mehrali, M.; Kazi, S.N.; Oon, C.S.; Metselaar, H.S.C. Experimental Investigation of Convective Heat Transfer Using Graphene Nanoplatelet Based Nanofluids under Turbulent Flow Conditions. Ind. Eng. Chem. Res. 2014, 53, 12455–12465. [Google Scholar] [CrossRef]
- Mehrali, M.; Sadeghinezhad, E.; Rosen, M.A.; Akhiani, A.R.; Latibari, S.T.; Mehrali, M.; Metselaar, H.S.C. Heat transfer and entropy generation for laminar forced convection flow of graphene nanoplatelets nanofluids in a horizontal tube. Int. Commun. Heat Mass Transf. 2015, 66, 23–31. [Google Scholar] [CrossRef]
- Agromayor, R.; Cabaleiro, D.; Pardinas, A.A.; Vallejo, J.P.; Fernandez-Seara, J.; Lugo, L. Heat Transfer Performance of Functionalized Graphene Nanoplatelet Aqueous Nanofluids. Materials 2016, 9, 455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrali, M.; Ghatkesar, M.K.; Pecnik, R. Full-spectrum volumetric solar thermal conversion via graphene/silver hybrid plasmonic nanofluids. Appl. Energy 2018, 224, 103–115. [Google Scholar] [CrossRef]
- Selvaraj, V.; Krishnan, H. Synthesis of graphene encased alumina and its application as nanofluid for cooling of heat-generating electronic devices. Powder Technol. 2020, 363, 665–675. [Google Scholar] [CrossRef]
- Rueda-García, D.; Rodríguez-Laguna, M.D.R.; Chávez-Angel, E.; Dubal, D.P.; Cabán-Huertas, Z.; Benages-Vilau, R.; Gómez-Romero, P. From thermal to electroactive graphene nanofluids. Energies 2019, 12, 4545. [Google Scholar] [CrossRef] [Green Version]
- Bahiraei, M.; Heshmatian, S. Graphene family nanofluids: A critical review and future research directions. Energy Convers. Manag. 2019, 196, 1222–1256. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Languri, E.M.; Nunna, M.R. Effect of particle size and viscosity on thermal conductivity enhancement of graphene oxide nanofluid. Int. Commun. Heat Mass Transf. 2016, 76, 308–315. [Google Scholar] [CrossRef]
- Ijam, A.; Saidur, R.; Ganesan, P.; Golsheikh, A.M. Stability, thermo-physical properties, and electrical conductivity of graphene oxide-deionized water/ethylene glycol based nanofluid. Int. J. Heat Mass Transf. 2015, 87, 92–103. [Google Scholar] [CrossRef]
- Said, Z.; Abdelkareem, M.A.; Rezk, H.; Nassef, A.M.; Atwany, H.Z. Stability, thermophysical and electrical properties of synthesized carbon nanofiber and reduced-graphene oxide-based nanofluids and their hybrid along with fuzzy modeling approach. Powder Technol. 2020, 364, 795–809. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Lin, Y.; Feng, M.; Wu, Q. Stability, thermal conductivity, and rheological properties of controlled reduced graphene oxide dispersed nanofluids. Appl. Therm. Eng. 2017, 119, 132–139. [Google Scholar] [CrossRef]
- Kamatchi, R.; Venkatachalapathy, S.; Srinivas, B.A. Synthesis, stability, transport properties, and surface wettability of reduced graphene oxide/water nanofluids. Int. J. Therm. Sci. 2015, 97, 17–25. [Google Scholar] [CrossRef]
- Pavía, M.; Alajami, K.; Estellé, P.; Desforges, A.; Vigolo, B. A critical review on thermal conductivity enhancement of graphene-based nanofluids. Adv. Colloid Interface Sci. 2021, 294, 102452. [Google Scholar] [CrossRef] [PubMed]
- Mandhare, H.; Barai, D.P.; Bhanvase, B.A.; Saharan, V.K. Preparation and thermal conductivity investigation of reduced graphene oxide-ZnO nanocomposite-based nanofluid synthesised by ultrasound-assisted method. Mater. Res. Innov. 2020, 24, 433–441. [Google Scholar] [CrossRef]
- Chawhan, S.S.; Barai, D.P.; Bhanvase, B.A. Sonochemical preparation of rGO-SnO2 nanocomposite and its nanofluids: Characterization, thermal conductivity, rheological and convective heat transfer investigation. Mater. Today Commun. 2020, 23, 101148. [Google Scholar] [CrossRef]
- Mehrali, M.; Sadeghinezhad, E.; Akhiani, A.R.; Latibari, S.T.; Talebian, S.; Dolatshahi-Pirouz, A.; Metselaar, H.S.C.; Mehrali, M. An ecofriendly graphene-based nanofluid for heat transfer applications. J. Clean. Prod. 2016, 137, 555–566. [Google Scholar] [CrossRef]
- Chen, P.; Harmand, S.; Szunerits, S.; Boukherroub., R. Evaporation behavior of PEGylated graphene oxide nanofluid droplets on heated substrate. Int. J. Therm. Scie. 2019, 135, 445–458. [Google Scholar]
- Hamze, S.; Cabaleiro, D.; Estellé, P. EGraphene-based nanofluids: A comprehensive review about rheological behavior and dynamic viscosity. J. Mol. Liq. 2021, 325, 115207. [Google Scholar] [CrossRef]
- Kotov, N.A. Carbon sheet solutions. Nature 2006, 442, 254–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, H.-Y.; Guo, Z.-X.; Xiang, H.; Gong, X.-G. Layer and size dependence of thermal conductivity in multilayer graphene nanoribbons. Phys. Lett. A 2012, 376, 525–528. [Google Scholar] [CrossRef] [Green Version]
- Yarmand, H.; Gharehkhani, S.; Shirazi, S.F.S.; Amiri, A.; Alehashem, M.S.; Dahari, M.; Kazi, S.N. Experimental investigation of thermo-physical properties, convective heat transfer and pressure drop of functionalized graphene nanoplatelets aqueous nanofluid in a square heated pipe. Energy Convers. Manag. 2016, 114, 38–49. [Google Scholar] [CrossRef]
- Yarmand, H.; Gharehkhani, S.; Shirazi, S.F.S.; Goodarzi, M.; Amiri, A.; Sarsam, W.S.; Alehashem, M.S.; Dahari, M.; Kazi, S.N. Study of synthesis, stability and thermo-physical properties of graphene nanoplatelet/platinum hybrid nanofluid. Int. Commun. Heat Mass Transf. 2016, 77, 15–21. [Google Scholar] [CrossRef]
- Mehrali, M.; Sadeghinezhad, E.; Latibari, S.T.; Mehrali, M.; Togun, H.; Zubir, M.N.M.; Kazi, S.N.; Metselaar, H.S.C. Preparation, characterization, viscosity, and thermal conductivity of nitrogen-doped graphene aqueous nanofluids. J. Mater. Sci. 2014, 49, 7156–7171. [Google Scholar] [CrossRef]
- Demirkır, Ç.; Ertürk, H. Rheological and thermal characterization of graphene-water nanofluids: Hysteresis phenomenon. Int. J. Heat Mass Transf. 2020, 149, 3–11. [Google Scholar] [CrossRef]
- Sarsam, W.S.; Amiri, A.; Kazi, S.N.; Badarudin, A. Stability and thermophysical properties of non-covalently functionalized graphene nanoplatelets nanofluids. Energy Convers. Manag. 2016, 116, 101–111. [Google Scholar] [CrossRef]
- Wusiman, K.; Jeong, H.; Tulugan, K.; Afrianto, H.; Chung, H. Thermal performance of multi-walled carbon nanotubes (MWCNTs) in aqueous suspensions with surfactants SDBS and SDS. Int. Commun. Heat Mass Transf. 2013, 41, 28–33. [Google Scholar] [CrossRef]
- Seong, H.J.; Kim, G.N.; Jeon, J.H.; Jeong, H.M.; Noh, J.P.; Kim, Y.J.; Kim, H.J.; Huh, S.C. Experimental study on characteristics of grinded graphene nanofluids with surfactants. Materials 2018, 11, 950. [Google Scholar] [CrossRef] [Green Version]
- Ilyas, S.U.; Ridha, S.; Kareem, F.A.A. Dispersion stability and surface tension of SDS-Stabilized saline nanofluids with graphene nanoplatelets. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 592. [Google Scholar] [CrossRef]
- Shazali, S.S.; Amiri, A.; Zubir, M.N.M.; Rozali, S.; Zabri, M.Z.; Sabri, M.F.M.; Soleymaniha, M. Investigation of the thermophysical properties and stability performance of non-covalently functionalized graphene nanoplatelets with Pluronic P-123 in different solvents. Mater. Chem. Phys. 2018, 206, 94–102. [Google Scholar] [CrossRef]
- Ghozatloo, A.; Shariaty-Niasar, M.; Rashidi, A.M. Preparation of nanofluids from functionalized Graphene by new alkaline method and study on the thermal conductivity and stability. Int. Commun. Heat Mass Transf. 2013, 42, 89–94. [Google Scholar] [CrossRef]
- Baby, T.T.; Ramaprabhu, S. Enhanced convective heat transfer using graphene dispersed nanofluids. Nanoscale Res. Lett. 2011, 6, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sezer, N.; Koç, M. Stabilization of the aqueous dispersion of carbon nanotubes using different approaches. Therm. Sci. Eng. Prog. 2018, 8, 411–417. [Google Scholar] [CrossRef]
- Barai, D.P.; Bhanvase, B.A.; Saharan, V.K. Reduced Graphene Oxide-Fe3O4 Nanocomposite Based Nanofluids: Study on Ultrasonic Assisted Synthesis, Thermal Conductivity, Rheology, and Convective Heat Transfer. Ind. Eng. Chem. Res. 2019, 58, 8349–8369. [Google Scholar] [CrossRef]
- Deosarkar, M.P.; Pawar, S.M.; Bhanvase, B.A. In situ sonochemical synthesis of Fe3O4–graphene nanocomposite for lithium rechargeable batteries. Chem. Eng. Process. Process Intensif. 2014, 83, 49–55. [Google Scholar] [CrossRef]
- Kumar, A.; Rout, L.; Dhaka, R.S.; Samal, S.L.; Dash, P. Design of a graphene oxide-SnO2 nanocomposite with superior catalytic efficiency for the synthesis of β-enaminones and β-enaminoesters. RSC Adv. 2015, 5, 39193–39204. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Shende, T.P.; Sonawane, S.H. A review on graphene–TiO2 and doped graphene–TiO2 nanocomposite photocatalyst for water and wastewater treatment. Environ. Technol. Rev. 2017, 6, 1–14. [Google Scholar] [CrossRef]
- Baby, T.T.; Sundara, R. Synthesis and transport properties of metal oxide decorated graphene dispersed nanofluids. J. Phys. Chem. C 2011, 115, 8527–8533. [Google Scholar] [CrossRef]
- Batmunkh, M.; Tanshen, M.R.; Nine, M.J.; Myekhlai, M.; Choi, H.; Chung, H.; Jeong, H. Thermal Conductivity of TiO2 Nanoparticles Based Aqueous Nanofluids with an Addition of a Modified Silver Particle. Ind. Eng. Chem. Res. 2014, 53, 8445–8451. [Google Scholar] [CrossRef]
- Yarmand, H.; Gharehkhani, S.; Ahmadi, G.; Shirazi, S.F.S.; Baradaran, S.; Montazer, E.; Zubir, M.N.M.; Alehashem, M.S.; Kazi, S.N.; Dahari, M. Graphene nanoplatelets-silver hybrid nanofluids for enhanced heat transfer. Energy Convers. Manag. 2015, 100, 419–428. [Google Scholar] [CrossRef]
- Baby, T.T.; Ramaprabhu, S. Synthesis and nanofluid application of silver nanoparticles decorated graphene. J. Mater. Chem. 2011, 21, 9702–9709. [Google Scholar] [CrossRef]
- Gu, Y.; Xu, S.; Wu, X. Thermal conductivity enhancements and viscosity properties of water based Nanofluid containing carbon nanotubes decorated with Ag nanoparticles. Heat Mass Transf. 2018, 54, 1847–1852. [Google Scholar] [CrossRef]
- Ba, T.L.; Mahian, O.; Wongwises, S.; Szilágyi, I.M. Review on the recent progress in the preparation and stability of graphene-based nanofluids. J. Therm. Anal. Calorim. 2020, 142, 1145–1172. [Google Scholar]
- Esfahani, M.R.; Languri, E.M. Exergy analysis of a shell-and-tube heat exchanger using graphene oxide nanofluids. Exp. Therm. Fluid Sci. 2017, 83, 100–106. [Google Scholar] [CrossRef]
- Dissolving Graphite to Get Graphene-Liquid Phase Exfoliation. Available online: www.youtube.com/watch?v=D9LePQUV6gc (accessed on 24 February 2022).
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.W.; Voon, C.H. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Shahsavar, A.; Salimpour, M.R.; Saghafian, M.; Shafii, M.B. An experimental study on the effect of ultrasonication on thermal conductivity of ferrofluid loaded with carbon nanotubes. Thermochim. Acta 2015, 617, 102–110. [Google Scholar] [CrossRef]
- Nasiri, A.; Shariaty-Niasar, M.; Rashidi, A.; Amrollahi, A.; Khodafarin, R. Effect of dispersion method on thermal conductivity and stability of nanofluid. Exp. Therm. Fluid Sci. 2011, 35, 717–723. [Google Scholar] [CrossRef]
- Suresh, S.; Venkitaraj, K.P.; Selvakumar, P.; Chandrasekar, M. Synthesis of Al2O3–Cu/water hybrid nanofluids using two step method and its thermo physical properties. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 388, 41–48. [Google Scholar] [CrossRef]
- Martínez, V.A.; Lozano-Steinmetz, F.; Vasco, D.A.; Zapata, P.A.; Chi-Durán, I.; Singh, D.P. Thermal characterization and stability analysis of aqueous ZnO-based nanofluids numerically implemented in microchannel heat sinks. Therm. Sci. Eng. Prog. 2021, 22, 142–149. [Google Scholar] [CrossRef]
- IAPWS. Revised Release on the IAPWS Formulation 1985 for the Thermal Conductivity of Ordinary Water Substance. Available online: www.iapws.org (accessed on 24 February 2022).
- Schmidt, A.J.; Chiesa, M.; Torchinsky, D.H.; Johnson, J.A.; Nelson, K.A.; Chen, G. Thermal conductivity of nanoparticle suspensions in insulating media measured with a transient optical grating and a hotwire. J. Appl. Phys. 2008, 103, 083529. [Google Scholar] [CrossRef]
- Kim, D.; Jeong, S.; Moon, J. Synthesis of silver nanoparticles using the polyol process and the influence of precursor injection. Nanotechnology 2006, 17, 4019–4024. [Google Scholar] [CrossRef]
- Aybar, H.Ş.; Sharifpur, M.; Azizian, M.R.; Mehrabi, M.; Meyer, J.P. A Review of Thermal Conductivity Models for Nanofluids. Heat Transf. Eng. 2015, 36, 1085–1110. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.J.; Zheng, R.T.; Gao, J.W.; Chen, G. Heat conduction mechanisms in nanofluids and suspensions. Nano Today 2012, 7, 124–136. [Google Scholar] [CrossRef] [Green Version]
- Martínez, V.A.; Vasco, D.A.; García-Herrera, C.M. Transient measurement of the thermal conductivity as a tool for the evaluation of the stability of nanofluids subjected to a pressure treatment. Int. Commun. Heat Mass Transf. 2018, 91, 234–238. [Google Scholar] [CrossRef]
- Shende, R.; Sundara, R. Nitrogen doped hybrid carbon based composite dispersed nanofluids as working fluid for low-temperature direct absorption solar collectors. Sol. Energy Mater. Sol. Cells. 2015, 114, 9–16. [Google Scholar] [CrossRef]
- Palm, S.J.; Roy, G.; Nguyen, C.T. Heat transfer enhancement with the use of nanofluids in radial flow cooling systems considering temperature-dependent properties. Appl. Therm. Eng. 2006, 26, 2209–2218. [Google Scholar] [CrossRef]
- Jin, C.; Wu, Q.; Yang, G.; Zhang, H.; Zhong, Y. Investigation on hybrid nanofluids based on carbon nanotubes filled with metal nanoparticles: Stability, thermal conductivity, and viscosity. Powder Technol. 2021, 389, 1–10. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, H.; Sasmito, A.P.; Mujumdar, A.S. Measurement and modeling of thermal conductivity of graphene nanoplatelet water and ethylene glycol base nanofluids. Int. J. Heat Mass Transf. 2018, 123, 97–109. [Google Scholar] [CrossRef]
- Ranjbarzadeh, R.; Akhgar, A.; Musivand, S.; Afrand, M. Effects of graphene oxide-silicon oxide hybrid nanomaterials on rheological behavior of water at various time durations and temperatures: Synthesis, preparation and stability. Powder Technol. 2018, 335, 375–387. [Google Scholar] [CrossRef]
- Sadeghinezhad, E.; Mehrali, M.; Akhiani, A.R.; Latibari, S.T.; Dolatshahi-Pirouz, A.; Metselaar, H.S.C.; Mehrali, M. Experimental study on heat transfer augmentation of graphene based ferrofluids in presence of magnetic field. Appl. Therm. Eng. 2017, 114, 415–427. [Google Scholar] [CrossRef]
- Tesfai, W.; Singh, P.; Shatilla, Y.; Iqbal, M.Z.; Abdala, A.A. Rheology and microstructure of dilute graphene oxide suspension. J. Nanoparticle Res. 2013, 15, 1989. [Google Scholar] [CrossRef]
- IAPWS. Revised Release on the IAPWS Formulation 1995 for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use (International Association for the Properties of Water and Steam. Available online: www.iapws.org (accessed on 24 February 2022).
- Hajjar, Z.; morad Rashidi, A.; Ghozatloo, A. Enhanced thermal conductivities of graphene oxide nanofluids. Int. Commun. Heat Mass Transf. 2014, 57, 128–131. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Desgranges, F.; Galanis, N.; Roy, G.; Thierry, M.; Boucher, S.; Angue-Mintsa, H. Viscosity data for Al2O3–water nanofluid—hysteresis: Is heat transfer enhancement using nanofluids reliable? Int. J. Therm. Sci. 2008, 47, 103–111. [Google Scholar] [CrossRef]






| Parameter | 0.01 M | 0.1 M | 1.0 M |
|---|---|---|---|
| A (W/mK) | 0.459 | 0.499 | 0.657 |
| B (min) | 6.208 | 5.945 | 4.472 |
| C (W/mK) | 0.595 | 0.635 | 0.647 |
| S.E. (W/mK) | 0.012 | 0.008 | 0.032 |
| R | 0.993 | 0.997 | 0.968 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano-Steinmetz, F.; Martínez, V.A.; Vasco, D.A.; Sepúlveda-Mualin, A.; Singh, D.P. The Effect of Ag-Decoration on rGO/Water Nanofluid Thermal Conductivity and Viscosity. Nanomaterials 2022, 12, 1095. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12071095
Lozano-Steinmetz F, Martínez VA, Vasco DA, Sepúlveda-Mualin A, Singh DP. The Effect of Ag-Decoration on rGO/Water Nanofluid Thermal Conductivity and Viscosity. Nanomaterials. 2022; 12(7):1095. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12071095
Chicago/Turabian StyleLozano-Steinmetz, Felipe, Victor A. Martínez, Diego A. Vasco, Alonso Sepúlveda-Mualin, and Dinesh Patrap Singh. 2022. "The Effect of Ag-Decoration on rGO/Water Nanofluid Thermal Conductivity and Viscosity" Nanomaterials 12, no. 7: 1095. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12071095