Microwave-Assisted Synthesis of N/TiO2 Nanoparticles for Photocatalysis under Different Irradiation Spectra
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
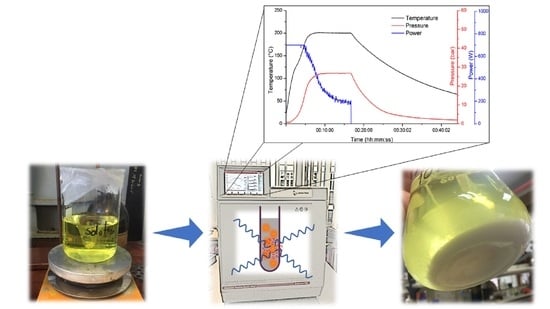
2.2. Microwave-Assisted Synthesis Parameters
2.3. N/TiO2 Microwave-Assisted Synthesis
2.4. Photocatalysts Characterization
2.5. Photocatalytic Activity Evaluation
3. Results & Discussion
3.1. Characterization of N/TiO2 for Optimization of the Reaction Time and Temperature
3.2. Photocatalysis of N/TiO2 for Optimization of the Reaction Time and Temperature
3.3. Bulk Characterization of N/TiO2 for Optimization of the N Doping Amount
3.4. Surface Specific Characterization of N/TiO2 for Optimization of the N Doping
3.5. Photocatalytic Performance Experiment for N/TiO2 Samples for Optimization of N Content
3.6. Determining the Relation Adsorption/Photocatalytic Removal of CIP for Different Irradiation Sources
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, J.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Occurrence, fate and transformation of emerging contaminants in water: An overarching review of the field. Environ. Pollut. 2017, 231, 954–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morin-Crini, N.; Lichtfouse, E.; Fourmentin, M.; Ribeiro, A.R.L.; Noutsopoulos, C.; Mapelli, F.; Fenyvesi, É.; Vieira, M.G.A.; Picos-Corrales, L.A.; Moreno-Piraján, J.C.; et al. Remediation of Emerging Contaminants; Morin-Crini, N., Lichtfouse, E., Crini, G., Eds.; Springer: Cham, Switzerland, 2021; pp. 1–106. [Google Scholar] [CrossRef]
- Cui, N.; Zada, A.; Song, J.; Yang, Y.; Liu, M.; Wang, Y.; Wu, Y.; Qi, K.; Selvaraj, R.; Liu, S.Y.; et al. Plasmon-induced ZnO-Ag/AgCl photocatalyst for degradation of tetracycline hydrochloride. Desalin. Water Treat. 2022, 245, 247–254. [Google Scholar] [CrossRef]
- Zhang, Z.; Zada, A.; Cui, N.; Liu, N.; Liu, M.; Yang, Y.; Jiang, D.; Jiang, J.; Liu, S. Synthesis of ag loaded ZnO/BiOCl with high photocatalytic performance for the removal of antibiotic pollutants. Crystals 2021, 11, 981. [Google Scholar] [CrossRef]
- Krzeminski, P.; Tomei, M.C.; Karaolia, P.; Langenhoff, A.; Almeida, C.M.R.; Felis, E.; Gritten, F.; Andersen, H.R.; Fernandes, T.; Manaia, C.M.; et al. Performance of secondary wastewater treatment methods for the removal of contaminants of emerging concern implicated in crop uptake and antibiotic resistance spread: A review. Sci. Total Environ. 2019, 648, 1052–1081. [Google Scholar] [CrossRef] [Green Version]
- Kutuzova, A.; Dontsova, T.; Kwapinski, W. Application of TiO2-Based Photocatalysts to Antibiotics Degradation: Cases of Sulfamethoxazole, Trimethoprim and Ciprofloxacin. Catalysts 2021, 11, 728. [Google Scholar] [CrossRef]
- Radović Vučić, M.; Baošić, R.; Mitrović, J.; Petrović, M.; Velinov, N.; Kostić, M.; Bojić, A. Comparison of the advanced oxidation processes in the degradation of pharmaceuticals and pesticides in simulated urban wastewater: Principal component analysis and energy requirements. Process Saf. Environ. Prot. 2021, 149, 786–793. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Truppi, A.; Petronella, F.; Placido, T.; Striccoli, M.; Agostiano, A.; Curri, M.L.; Comparelli, R. Visible-light-active TiO2-based hybrid nanocatalysts for environmental applications. Catalysts 2017, 7, 100. [Google Scholar] [CrossRef]
- Šegota, S.; Ćurković, L.; Ljubas, D.; Svetličić, V.; Houra, I.F.; Tomašić, N. Synthesis, characterization and photocatalytic properties of sol-gel TiO2 films. Ceram. Int. 2011, 37, 1153–1160. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Shayegan, Z.; Lee, C.S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef] [Green Version]
- Zada, A.; Khan, M.; Hussain, Z.; Shah, M.I.A.; Ateeq, M.; Ullah, M.; Ali, N.; Shaheen, S.; Yasmeen, H.; Ali Shah, S.N.; et al. Extended visible light driven photocatalytic hydrogen generation by electron induction from g-C3N4nanosheets to ZnO through the proper heterojunction. Z. Phys. Chem. 2022, 236, 53–66. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Piątkowska, A.; Janus, M.; Szymański, K.; Mozia, S. C-,N- and S-Doped TiO2 Photocatalysts: A Review. Catalysts 2021, 11, 144. [Google Scholar] [CrossRef]
- Ding, W.; Li, W. A first principles study of the energetics and core level shifts of anion-doped TiO2 photocatalysts. Chin. J. Catal. 2014, 36, 181–187. [Google Scholar] [CrossRef]
- Brindha, A.; Sivakumar, T. Visible active N, S co-doped TiO2/graphene photocatalysts for the degradation of hazardous dyes. J. Photochem. Photobiol. A Chem. 2017, 340, 146–156. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Mozhiarasi, V.; Tayade, R.J. Nitrogen Doped Titanium Dioxide (N-TiO2): Synopsis of Synthesis Methodologies, Doping Mechanisms, Property Evaluation and Visible Light Photocatalytic Applications. Photochem 2021, 1, 371–410. [Google Scholar] [CrossRef]
- Gomes, J.; Lincho, J.; Domingues, E.; Quinta-Ferreira, R.; Martins, R. N–TiO2 Photocatalysts: A Review of Their Characteristics and Capacity for Emerging Contaminants Removal. Water 2019, 11, 373. [Google Scholar] [CrossRef] [Green Version]
- Suwannaruang, T.; Hildebrand, J.P.; Taffa, D.H.; Wark, M.; Kamonsuangkasem, K.; Chirawatkul, P.; Wantala, K. Visible light-induced degradation of antibiotic ciprofloxacin over Fe–N–TiO2 mesoporous photocatalyst with anatase/rutile/brookite nanocrystal mixture. J. Photochem. Photobiol. A Chem. 2020, 391, 112371. [Google Scholar] [CrossRef]
- Catauro, M.; Tranquillo, E.; Dal Poggetto, G.; Pasquali, M.; Dell’Era, A.; Ciprioti, S.V. Influence of the heat treatment on the particles size and on the crystalline phase of TiO2 synthesized by the sol-gel method. Materials 2018, 11, 2364. [Google Scholar] [CrossRef] [Green Version]
- Xi, J.; Zhang, Y.; Chen, X.; Hu, Y. A simple sol–gel hydrothermal method for the synthesis of defective TiO2 nanocrystals with excellent visible-light photocatalytic activity. Res. Chem. Intermed. 2020, 46, 2205–2214. [Google Scholar] [CrossRef]
- Gabelica, I.; Ćurković, L.; Mandić, V.; Panžić, I.; Ljubas, D.; Zadro, K. Rapid microwave-assisted synthesis of Fe3O4/SiO2/TiO2 core-2-layer-shell nanocomposite for photocatalytic degradation of ciprofloxacin. Catalysts 2021, 11, 1136. [Google Scholar] [CrossRef]
- Blanco-Vega, M.P.; Guzmán-Mar, J.L.; Villanueva-Rodríguez, M.; Maya-Treviño, L.; Garza-Tovar, L.L.; Hernández-Ramírez, A.; Hinojosa-Reyes, L. Photocatalytic elimination of bisphenol A under visible light using Ni-doped TiO2 synthesized by microwave assisted sol-gel method. Mater. Sci. Semicond. Process. 2017, 71, 275–282. [Google Scholar] [CrossRef]
- Mendiola-Alvarez, S.Y.; Guzmán-Mar, J.L.; Turnes-Palomino, G.; Maya-Alejandro, F.; Hernández-Ramírez, A.; Hinojosa-Reyes, L. UV and visible activation of Cr(III)-doped TiO2 catalyst prepared by a microwave-assisted sol–gel method during MCPA degradation. Environ. Sci. Pollut. Res. 2017, 24, 12673–12682. [Google Scholar] [CrossRef]
- Paluch, K.J.; McCabe, T.; Müller-Bunz, H.; Corrigan, O.I.; Healy, A.M.; Tajber, L. Formation and physicochemical properties of crystalline and amorphous salts with different stoichiometries formed between ciprofloxacin and succinic acid. Mol. Pharm. 2013, 10, 3640–3654. [Google Scholar] [CrossRef]
- Sanchis-Perucho, A.; Orts-Arroyo, M.; Camús-Hernández, J.; Rojas-Dotti, C.; Escrivà, E.; Lloret, F.; Martínez-Lillo, J. Hexahalorhenate(IV) salts of protonated ciprofloxacin: Antibiotic-based single-ion magnets. CrystEngComm 2021, 23, 8579–8587. [Google Scholar] [CrossRef]
- Golovnev, N.N.; Molokeev, M.S.; Lesnikov, M.K.; Atuchin, V.V. Two salts and the salt cocrystal of ciprofloxacin with thiobarbituric and barbituric acids: The structure and properties. J. Phys. Org. Chem. 2018, 31, e3773. [Google Scholar] [CrossRef] [Green Version]
- Commission Implementing Decision (EU) 2018/840 of 5 June 2018 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council and Repealing Commission Implementing Decision (EU) 2015/495 (Notified under Document C(2018) 3362). Off. J. Eur. Union 2018, L141, 9–12.
- Commission Implementing Decision (EU) 2020/1161 of 4 August 2020 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council (Notified under Document Number C(2020) 5205). Off. J. Eur. Union 2020, L257, 32–35.
- Malakootian, M.; Faraji, M.; Malakootian, M.; Nozari, M. Ciprofloxacin removal from aqueous media by adsorption process: A systematic review and meta-analysis. Desalin. Water Treat. 2021, 229, 252–282. [Google Scholar] [CrossRef]
- Do, M.T.; Stuckey, D.C. Fate and removal of Ciprofloxacin in an anaerobic membrane bioreactor (AnMBR). Bioresour. Technol. 2019, 289, 121683. [Google Scholar] [CrossRef]
- Thapa, R.; Maiti, S.; Rana, T.H.; Maiti, U.N.; Chattopadhyay, K.K. Anatase TiO2 nanoparticles synthesis via simple hydrothermal route: Degradation of Orange II, Methyl Orange and Rhodamine B. J. Mol. Catal. A Chem. 2012, 363–364, 223–229. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, X.; Xing, Z.; Yang, L. Synthesis and characterization of N-doped TiO2 and its enhanced visible-light photocatalytic activity. Arab. J. Chem. 2016, 9, S1706–S1711. [Google Scholar] [CrossRef]
- Sher Shah, M.S.A.; Park, A.R.; Zhang, K.; Park, J.H.; Yoo, P.J. Green synthesis of biphasic TiO2-reduced graphene oxide nanocomposites with highly enhanced photocatalytic activity. ACS Appl. Mater. Interfaces 2012, 4, 3893–3901. [Google Scholar] [CrossRef]
- Factorovich, M.; Guz, L.; Candal, R. N-TiO2: Chemical synthesis and photocatalysis. Adv. Phys. Chem. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- Švagelj, Z.; Mandić, V.; Ćurković, L.; Biošić, M.; Žmak, I.; Gaborardi, M. Titania-Coated alumina foam photocatalyst for memantine degradation derived by replica method and sol-gel reaction. Materials 2020, 13, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadam, A.N.; Dhabbe, R.S.; Kokate, M.R.; Gaikwad, Y.B.; Garadkar, K.M. Preparation of N doped TiO2 via microwave-assisted method and its photocatalytic activity for degradation of Malathion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Hao, K.; Dai, Y.; Song, L.; Yu, Q.; Yin, X.; Wang, Z. Enhanced Visible-Light Photocatalytic Activity by the Comprehensive Effects of Mesoporous and N-Doping at the Meso-N-TiO2 Nanocatalysts. ChemistrySelect 2021, 6, 6029–6036. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, Z.; Shi, H.; Xiao, T.; Yan, Z. Preparation of highly visible-light active N-doped TiO2 photocatalyst. J. Mater. Chem. 2010, 20, 5301–5309. [Google Scholar] [CrossRef]
- Barkul, R.P.; Koli, V.B.; Shewale, V.B.; Patil, M.K.; Delekar, S.D. Visible active nanocrystalline N-doped anatase TiO2 particles for photocatalytic mineralization studies. Mater. Chem. Phys. 2016, 173, 42–51. [Google Scholar] [CrossRef]
- Chung, K.H.; Kim, B.J.; Park, Y.K.; Kim, S.C.; Jung, S.C. Photocatalytic properties of amorphous n-doped TiO2 photocatalyst under visible light irradiation. Catalysts 2021, 11, 1010. [Google Scholar] [CrossRef]
- Madurai Ramakrishnan, V.; Sandberg, S.; Muthukumarasamy, N.; Kvamme, K.; Balraju, P.; Agilan, S.; Velauthapillai, D. Microwave-assisted solvothermal synthesis of worms-like TiO2 nanostructures in submicron regime as light scattering layers for dye-sensitized solar cells. Mater. Lett. 2019, 236, 747–751. [Google Scholar] [CrossRef]
- Russo, P.; Liang, R.; He, R.X.; Zhou, Y.N. Phase transformation of TiO2 nanoparticles by femtosecond laser ablation in aqueous solutions and deposition on conductive substrates. Nanoscale 2017, 9, 6167–6177. [Google Scholar] [CrossRef]
- Bakre, P.V.; Tilve, S.G.; Shirsat, R.N. Influence of N sources on the photocatalytic activity of N-doped TiO2. Arab. J. Chem. 2020, 13, 7637–7651. [Google Scholar] [CrossRef]
- Kocijan, M.; Ćurković, L.; Radošević, T.; Podlogar, M. Enhanced photocatalytic activity of hybrid rGO@TiO2/CN nanocomposite for organic pollutant degradation under solar light irradiation. Catalysts 2021, 11, 1023. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Kesler, V.G.; Pervukhina, N.V.; Zhang, Z. Ti 2p and O 1s core levels and chemical bonding in titanium-bearing oxides. J. Electron Spectrosc. Relat. Phenom. 2006, 152, 18–24. [Google Scholar] [CrossRef]
- Nolan, N.T.; Synnott, D.W.; Seery, M.K.; Hinder, S.J.; Van Wassenhoven, A.; Pillai, S.C. Effect of N-doping on the photocatalytic activity of sol-gel TiO2. J. Hazard. Mater. 2012, 211–212, 88–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: Designs, developments, and prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef] [PubMed]
- Suwannaruang, T.; Kidkhunthod, P.; Chanlek, N.; Soontaranon, S.; Wantala, K. High anatase purity of nitrogen-doped TiO2 nanorice particles for the photocatalytic treatment activity of pharmaceutical wastewater. Appl. Surf. Sci. 2019, 478, 1–14. [Google Scholar] [CrossRef]
- Kuo, C.L.; Chen, W.G.; Chen, T.Y. The electronic structure changes and the origin of the enhanced optical properties in N-doped anatase TiO2—A theoretical revisit. J. Appl. Phys. 2014, 116, 093709. [Google Scholar] [CrossRef]
- Hu, S.; Wang, A.; Li, X.; Löwe, H. Hydrothermal synthesis of well-dispersed ultrafine N-doped TiO2 nanoparticles with enhanced photocatalytic activity under visible light. J. Phys. Chem. Solids 2010, 71, 156–162. [Google Scholar] [CrossRef]
- Rizzo, L.; Meric, S.; Kassinos, D.; Guida, M.; Russo, F.; Belgiorno, V. Degradation of diclofenac by TiO2 photocatalysis: UV absorbance kinetics and process evaluation through a set of toxicity bioassays. Water Res. 2009, 43, 979–988. [Google Scholar] [CrossRef]









| Material Labeling | Parameter | Section |
|---|---|---|
| N/TiO2 150_10 | Temperature (from 150 to 200 °C) and time (from 10 to 30 min) | Microwave-assisted synthesis parameters (Section 2.2) |
| N/TiO2 200_10 | ||
| N/TiO2 200_20 | ||
| N/TiO2 200_30 | ||
| N/TiO2 0 | Nitrogen content (from 0 to 24 N/Ti molar ratio) | N/TiO2 microwave-assisted synthesis parameters (Section 2.3) |
| N/TiO2 1 | ||
| N/TiO2 2 | ||
| N/TiO2 4 | ||
| N/TiO2 12 | ||
| N/TiO2 24 |
| Material | SBET, m2 g−1 | Vpore, cm3 g−1 | Average Pore Diameter, nm |
|---|---|---|---|
| N/TiO2 150_10 | 172.4 | 0.260 | 5.46 |
| N/TiO2 200_10 | 185.3 | 0.326 | 6.68 |
| N/TiO2 200_20 | 187.8 | 0.342 | 6.91 |
| N/TiO2 200_30 | 179.2 | 0.330 | 7.02 |
| SBET, m2 g−1 | Vpore, cm3 g−1 | Average PoreDiameter, nm | |
|---|---|---|---|
| N/TiO2 0 | 215.04 | 0.424 | 7.25 |
| N/TiO2 1 | 144.40 | 0.273 | 7.26 |
| N/TiO2 2 | 185.29 | 0.326 | 6.68 |
| N/TiO2 4 | 158.09 | 0.295 | 7.19 |
| N/TiO2 12 | 139.17 | 0.297 | 8.00 |
| Degussa P25 | 48.14 | 0.196 | 13.69 |
| k1, (min−1) | R2 | |||||
|---|---|---|---|---|---|---|
| Lamp | I | II | III | I | II | III |
| N/TiO2 0 | 0.0380 | 0.0432 | 0.0021 | 0.9001 | 0.9023 | 0.9796 |
| N/TiO2 4 | 0.0406 | 0.0443 | 0.0028 | 0.8893 | 0.8928 | 0.9957 |
| N/TiO2 12 | 0.0432 | 0.0477 | 0.0053 | 0.8635 | 0.8843 | 0.9432 |
| N/TiO2 24 | 0.0425 | 0.0464 | 0.0055 | 0.8621 | 0.8673 | 0.9392 |
| Degussa P25 | 0.0588 | 0.0563 | - | 0.8562 | 0.8187 | - |
| k2, (L mg−1 min−1) | R2 | Removal Efficiency (ɳ) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lamp | I | II | III | I | II | III | I | II | III |
| N/TiO2 0 | 0.3905 | 0.5750 | 0.0028 | 0.9959 | 0.9909 | 0.9861 | 98.07% | 98.68% | 35.83% |
| N/TiO2 4 | 0.4798 | 0.6499 | 0.0044 | 0.9946 | 0.9886 | 0.9978 | 98.46% | 98.78% | 50.64% |
| N/TiO2 12 | 0.5881 | 0.9768 | 0.0088 | 0.9975 | 0.9869 | 0.9687 | 98.66% | 99.21% | 58.51% |
| N/TiO2 24 | 0.5376 | 0.7852 | 0.0091 | 0.9872 | 0.9974 | 0.9655 | 98.40% | 99.02% | 59.26% |
| Degussa P25 | 1.7868 | 1.2320 | - | 0.9907 | 0.9948 | - | 99.61% | 99.35% | 12.32% * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez Tobon, C.; Ljubas, D.; Mandić, V.; Panžić, I.; Matijašić, G.; Ćurković, L. Microwave-Assisted Synthesis of N/TiO2 Nanoparticles for Photocatalysis under Different Irradiation Spectra. Nanomaterials 2022, 12, 1473. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12091473
Sanchez Tobon C, Ljubas D, Mandić V, Panžić I, Matijašić G, Ćurković L. Microwave-Assisted Synthesis of N/TiO2 Nanoparticles for Photocatalysis under Different Irradiation Spectra. Nanomaterials. 2022; 12(9):1473. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12091473
Chicago/Turabian StyleSanchez Tobon, Camilo, Davor Ljubas, Vilko Mandić, Ivana Panžić, Gordana Matijašić, and Lidija Ćurković. 2022. "Microwave-Assisted Synthesis of N/TiO2 Nanoparticles for Photocatalysis under Different Irradiation Spectra" Nanomaterials 12, no. 9: 1473. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12091473