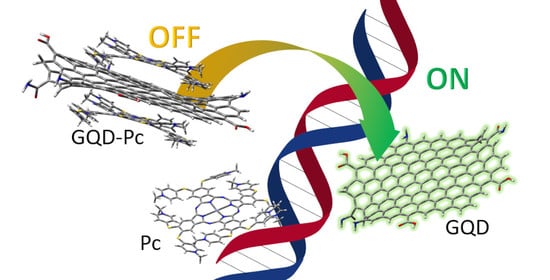
Graphene Quantum Dots and Phthalocyanines Turn-OFF-ON Photoluminescence Nanosensor for ds-DNA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Measurements
2.3. Synthesis of GQDs and Pcs
2.4. Preparation of GQD-Pc4 and GQD-Pc8 Nanohybrids
3. Results and Discussion
3.1. Characterization of GQDs
3.2. Characterization of Pc4 and Pc8
3.3. Titration of GQDs with Pcs
3.4. Sensing of DNA
3.5. Mechanistic Insight
3.6. Selectivity Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Srivastava, M.; Srivastava, N.; Mishra, P.K.; Malhotra, B.D. Prospects of nanomaterials-enabled biosensors for COVID-19 detection. Sci. Total Environ. 2021, 754, 142363. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Ji, C.Y.; Zhou, Y.Q.; Leblanc, R.M.; Peng, Z.L. Recent Developments of Carbon Dots in Biosensing: A Review. ACS Sens. 2020, 5, 2724. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Ren, J.S.; Qu, X.G. Carbon Nanomaterials and DNA: From Molecular Recognition to Applications. Acc. Chem. Res. 2016, 49, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.C.F.G.; Rocha, B.G.M.; Maçôas, E.M.S.; Marques, E.F.; Martinho, J.M.G. Combining metal nanoclusters and carbon nanomaterials: Opportunities and challenges in advanced nanohybrids. Adv. Colloid Interface 2022, 304, 102667. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, A.; Soriano, M.L.; Carrillo-Carrion, C.; Valcarcel, M. Semiconductor and carbon-based fluorescent nanodots: The need for consistency. Chem. Commun. 2016, 52, 1311. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020, 18, 703. [Google Scholar] [CrossRef] [Green Version]
- Zairov, R.R.; Dovzhenko, A.P.; Sarkanich, K.A.; Nizameev, I.R.; Luzhetskiy, A.V.; Sudakova, S.N.; Podyachev, S.N.; Burilov, V.A.; Vatsouro, I.M.; Vomiero, A.; et al. Single Excited Dual Band Luminescent Hybrid Carbon Dots-Terbium Chelate Nanothermometer. Nanomaterials 2021, 11, 3080. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Y.R.; Zhao, H.G.; Vomiero, A.; Li, R.G. Highly efficient ratiometric nanothermometers based on colloidal carbon quantum dots. J. Mater. Chem. B 2021, 9, 4111–4119. [Google Scholar] [CrossRef]
- Cui, X.; Zhu, L.; Wu, J.; Hou, Y.; Wang, P.Y.; Wang, Z.N.; Yang, M. A fluorescent biosensor based on carbon dots-labeled oligodeoxyribonucleotide and graphene oxide for mercury(II) detection. Biosens. Bioelectron. 2015, 63, 506. [Google Scholar] [CrossRef]
- Zhang, S.R.; Wang, Q.; Tian, G.H.; Ge, H.G. A fluorescent turn-off/on method for detection of Cu2+ and oxalate using carbon dots as fluorescent probes in aqueous solution. Matter. Lett. 2014, 115, 233. [Google Scholar] [CrossRef]
- Achadu, O.J.; Nyokong, T. Fluorescence “turn-ON” nanosensor for cyanide ion using supramolecular hybrid of graphene quantum dots and cobalt pyrene-derivatized phthalocyanine. Dye. Pigment. 2019, 160, 328. [Google Scholar] [CrossRef]
- Zhao, H.X.; Liu, L.Q.; Liu, Z.D.; Wang, Y.; Zhao, X.J.; Huang, C.Z. Highly selective detection of phosphate in very complicated matrixes with an off–on fluorescent probe of europium-adjusted carbon dots. Chem. Commun. 2011, 47, 2604. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Maruthapandi, M.; Saravanan, A.; Natan, M.; Jacobi, G.; Banin, E.; Gedanken, A. Carbon Dots for Heavy-Metal Sensing, pH-Sensitive Cargo Delivery, and Antibacterial Applications. ACS Appl. Nano Mater. 2020, 3, 11777–11790. [Google Scholar] [CrossRef]
- Pang, S.; Zhang, Y.; Wu, C.; Feng, S. Fluorescent carbon dots sensor for highly sensitive detection of guanine. Sens. Actuators B 2016, 222, 857. [Google Scholar] [CrossRef]
- Crista, D.M.A.; Mello, G.P.C.; Shevchuk, O.; Sendão, R.M.S.; Simões, E.F.C.; Leitão, J.M.M.; Silva, L.P.d.; Silva, J.C.G.E.d. 3-Hydroxyphenylboronic Acid-Based Carbon Dot Sensors for Fructose Sensing. J. Fluoresc. 2019, 29, 265. [Google Scholar] [CrossRef]
- Achadu, O.J.; Nyokong, T. Graphene quantum dots decorated with maleimide and zinc tetramaleimido-phthalocyanine: Application in the design of “OFF-ON” fluorescence sensors for biothiols. Talanta 2017, 166, 15. [Google Scholar] [CrossRef] [PubMed]
- Achadu, O.J.; Nyokong, T. Application of graphene quantum dots decorated with TEMPO-derivatized zinc phthalocyanine as novel nanoprobes: Probing the sensitive detection of ascorbic acid. New J. Chem. 2016, 40, 8727. [Google Scholar] [CrossRef]
- Huanga, S.; Wanga, L.; Zhua, F.; Sua, W.; Shenga, J.; Huang, C.; Xiao, Q. A ratiometric nanosensor based on fluorescent carbon dots for label-free and highly selective recognition of DNA. RSC Adv. 2015, 5, 44587. [Google Scholar] [CrossRef]
- Alarfaj, N.A.; El-Tohamy, M.F.; Oraby, H.F. CA 19-9 Pancreatic Tumor Marker Fluorescence Immunosensing Detection via Immobilized Carbon Quantum Dots Conjugated Gold Nanocomposite. Int. J. Mol. Sci. 2018, 19, 1162. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, S.; Salimi, A.; Hamd-Ghadareh, S.; Fathi, F.; Soleimani, F. A FRET immunosensor for sensitive detection of CA 15-3 tumor marker in human serum sample and breast cancer cells using antibody functionalized luminescent carbon-dots and AuNPs-dendrimer aptamer as donor-acceptor pair. Anal. Biochem. 2018, 557, 18. [Google Scholar] [CrossRef] [PubMed]
- Hamd-Ghadareh, S.; Salimi, A.; Fathi, F.; Bahrami, S. An amplified comparative fluorescence resonance energy transfer immunosensing of CA125 tumor marker and ovarian cancer cells using green and economic carbon dots for bio-applications in labeling, imaging and sensing. Biosens. Bioelectron. 2017, 96, 308. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.J.; Song, Y.B.; Zhao, X.H.; Shao, J.R.; Zhang, J.H.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355. [Google Scholar] [CrossRef]
- Gan, Z.X.; Xu, H.; Hao, Y.L. Mechanism for excitation-dependent photoluminescence from graphene quantum dots and other graphene oxide derivates: Consensus, debates and challenges. Nanoscale 2016, 8, 7794. [Google Scholar] [CrossRef]
- Sk, M.A.; Ananthanarayanan, A.; Huang, L.; Lim, K.H.; Chen, P. Revealing the tunable photoluminescence properties of graphene quantum dots. J. Mater. Chem. C 2014, 2, 6954. [Google Scholar] [CrossRef]
- Santos, C.I.M.; Mariz, I.F.A.; Pinto, S.N.; Gonçalves, G.; Bdikin, I.; Marques, P.A.A.P.; Neves, M.G.P.M.S.; Martinho, J.M.G.; Maçôas, E.M.S. Selective two-photon absorption in carbon dots: A piece of the photoluminescence emission puzzle. Nanoscale 2018, 10, 12505. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Dong, H.F.; Yang, Z.; Zhong, X.M.; Chen, Y.; Dai, W.H.; Zhang, X.J. Aptamer-Conjugated Graphene Quantum Dots/Porphyrin Derivative Theranostic Agent for Intracellular Cancer-Related MicroRNA Detection and Fluorescence-Guided Photothermal/Photodynamic Synergetic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 159. [Google Scholar] [CrossRef]
- Santos, C.I.M.; Rodriguez-Perez, L.; Goncalves, G.; Dias, C.J.; Monteiro, F.; Faustino, M.D.F.; Vieira, S.I.; Helguero, L.A.; Herranz, M.A.; Martin, N.; et al. Enhanced Photodynamic Therapy Effects of Graphene Quantum Dots Conjugated with Aminoporphyrins. ACS Appl. Nano Mater. 2021, 4, 13079. [Google Scholar] [CrossRef]
- Santos, C.I.M.; Rodriguez-Perez, L.; Goncalves, G.; Pinto, S.N.; Melle-Franco, M.; Marques, P.; Faustino, M.A.F.; Herranz, M.A.; Martin, N.; Neves, M.; et al. Novel hybrids based on graphene quantum dots covalently linked to glycol corroles for multiphoton bioimaging. Carbon 2020, 166, 164. [Google Scholar] [CrossRef]
- Achadu, O.J.; Uddin, I.; Nyokong, T. Fluorescence behavior of nanoconjugates of graphene quantum dots and zinc phthalocyanines. J. Photochem. Photobiol. A Chem. 2016, 317, 12. [Google Scholar] [CrossRef]
- Openda, Y.I.; Sen, P.; Managa, M.; Nyokong, T. Acetophenone substituted phthalocyanines and their graphene quantum dots conjugates as photosensitizers for photodynamic antimicrobial chemotherapy against Staphylococcus aureus. Photodiagnosis Photodyn. Ther. 2020, 29, 101607. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.H.; Noh, S.H.; Kim, T.-H.; Lee, W.J.; Yi, S.-C. A graphene quantum dot/phthalocyanine conjugate: A synergistic catalyst for the oxygen reduction reaction. RSC Adv. 2017, 7, 26113. [Google Scholar] [CrossRef] [Green Version]
- Nyokong, T. Effects of substituents on the photochemical and photophysical properties of main group metal phthalocyanines. Coord. Chem. Rev. 2007, 251, 1707. [Google Scholar] [CrossRef]
- Lourenco, L.M.O.; Pereira, P.M.R.; Maciel, E.; Valega, M.; Domingues, F.M.J.; Domingues, M.R.M.; Neves, M.; Cavaleiro, J.A.S.; Fernandes, R.; Tome, J.P.C. Amphiphilic phthalocyanine-cyclodextrin conjugates for cancer photodynamic therapy. Chem. Commun. 2014, 50, 8363. [Google Scholar] [CrossRef]
- Lo, P.C.; Rodriguez-Morgade, M.S.; Pandey, R.K.; Ng, D.K.P.; Torres, T.; Dumoulin, F. The unique features and promises of phthalocyanines as advanced photosensitisers for photodynamic therapy of cancer. Chem. Soc. Rev. 2020, 49, 1041. [Google Scholar] [CrossRef]
- Ribeiro, C.P.S.; Lourenco, L.M.O. Overview of cationic phthalocyanines for effective photoinactivation of pathogenic microorganisms. J. Photochem. Photobiol. C Photochem. Rev. 2021, 48, 100422. [Google Scholar] [CrossRef]
- Gamelas, S.R.D.; Gomes, A.T.P.C.; Faustino, M.A.F.; Tome, A.C.; Tome, J.P.C.; Almeida, A.; Lourenco, L.M.O. Photoinactivation of Escherichia coli with Water-Soluble Ammonium-Substituted Phthalocyanines. ACS Appl. Bio. Mater. 2020, 3, 4044. [Google Scholar] [CrossRef]
- Kumar, A.; Vashistha, V.K.; Das, D.K. Recent development on metal phthalocyanines based materials for energy conversion and storage applications. Coord. Chem. Rev. 2021, 431, 213678. [Google Scholar] [CrossRef]
- Zhou, R.; Josse, F.; Gopel, W.; Ozturk, Z.Z.; Bekaroglu, O. Phthalocyanines as sensitive materials for chemical sensors. Appl. Organomet. Chem. 1996, 10, 557. [Google Scholar] [CrossRef]
- Zairov, R.R.; Yagodin, A.V.; Khrizanforov, M.; Martynov, A.G.; Nizameev, I.R.; Syakaev, V.V.; Gubaidullin, A.T.; Kornev, T.; Kaman, O.; Budnikova, Y.H.; et al. Unusual magnetic relaxation behavior of hydrophilic colloids based on gadolinium(III) octabutoxyphthalocyaninate. J. Nanopart. Res. 2019, 21, 12. [Google Scholar] [CrossRef]
- D’Souza, F.; Ito, O. Supramolecular donor–acceptor hybrids of porphyrins/phthalocyanines with fullerenes/carbon nanotubes: Electron transfer, sensing, switching, and catalytic applications. Chem. Commun. 2009, 33, 4913. [Google Scholar] [CrossRef] [PubMed]
- Bottari, G.; Torre, G.d.l.; Torres, T. Phthalocyanine–Nanocarbon Ensembles: From Discrete Molecular and Supramolecular Systems to Hybrid Nanomaterials. Acc. Chem. Res. 2015, 48, 900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matshitse, R.; Sekhosana, K.E.; Achadu, O.J.; Nyokong, T. Characterization and physicochemical studies of the conjugates of graphene quantum dots with differently charged zinc phthalocyanines. J. Coord. Chem. 2017, 70, 3308. [Google Scholar] [CrossRef]
- Matshitse, R.; Nyokong, T. Singlet Oxygen Generating Properties of Different Sizes of Charged Graphene Quantum Dot Nanoconjugates with a Positively Charged Phthalocyanine. J. Fluoresc. 2018, 28, 827–838. [Google Scholar] [CrossRef]
- Fomo, G.; Achadu, O.J.; Nyokong, T. One-pot synthesis of graphene quantum dots-phthalocyanines supramolecular hybrid and the investigation of their photophysical properties. J. Mater. Sci. 2018, 53, 538. [Google Scholar] [CrossRef]
- Vikrant, K.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.H.; Deep, A. Nanomaterials as efficient platforms for sensing DNA. Biomaterials 2019, 214, 119215. [Google Scholar] [CrossRef]
- Kala, D.; Sharma, T.K.; Gupta, S.; Saini, R.V.; Saini, A.K.; Alsanie, W.F.; Thakur, V.K.; Kaushal, A. Development of paper-based DNA sensor for detection of O. tsutsugamushi using sustainable GQDs@AuNPs nanocomposite. Chemosphere 2022, 300, 134428. [Google Scholar] [CrossRef]
- Pereira, J.B.; Carvalho, E.F.A.; Faustino, M.A.F.; Fernandes, R.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Gomes, N.C.M.; Cunha, A.; Almeida, A.; Tome, J.P.C. Phthalocyanine Thio-Pyridinium Derivatives as Antibacterial Photosensitizers. Photochem. Photobiol. Phys. Rev. B 2012, 88, 537. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J.J.P.R.B. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. 2000, 61, 14095. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Chizhik, A.M.; Karedla, N.; Dekaliuk, M.O.; Gregor, I.; Schuhmann, H.; Seibt, M.; Bodensiek, K.; Schaap, I.A.T.; Schulz, O.; et al. Photoluminescence of Carbon Nanodots: Dipole Emission Centers and Electron-Phonon Coupling. Nano Lett. 2014, 14, 5656–5661. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago, A.M.; Santos, C.I.M.; Lourenço, L.M.O.; Mariz, I.F.A.; Tomé, J.P.C.; Maçôas, E. Graphene Quantum Dots and Phthalocyanines Turn-OFF-ON Photoluminescence Nanosensor for ds-DNA. Nanomaterials 2022, 12, 1892. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12111892
Santiago AM, Santos CIM, Lourenço LMO, Mariz IFA, Tomé JPC, Maçôas E. Graphene Quantum Dots and Phthalocyanines Turn-OFF-ON Photoluminescence Nanosensor for ds-DNA. Nanomaterials. 2022; 12(11):1892. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12111892
Chicago/Turabian StyleSantiago, Ana M., Carla I. M. Santos, Leandro M. O. Lourenço, Inês F. A. Mariz, João P. C. Tomé, and Ermelinda Maçôas. 2022. "Graphene Quantum Dots and Phthalocyanines Turn-OFF-ON Photoluminescence Nanosensor for ds-DNA" Nanomaterials 12, no. 11: 1892. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12111892