A Novel Turn-On Fluorescent Sensor Based on Sulfur Quantum Dots and MnO2 Nanosheet Architectures for Detection of Hydrazine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
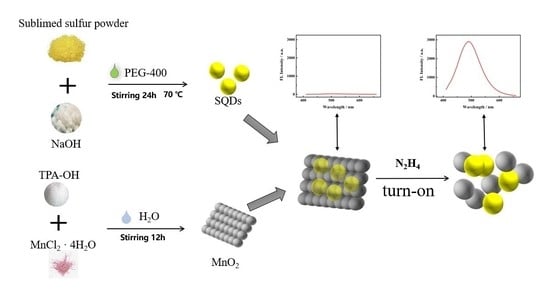
2.3. Synthesis of SQDs and MnO2 NS
2.4. The SQDs@MnO2 NS Fluorescent Probe Detection N2H4
2.5. Detection of Actual Samples
3. Results
3.1. Characteristics of SQDs, MnO2 NS, SQDs@MnO2
3.2. Optimization of Experimental Parameters
3.3. Fluorescence Spectra Analysis of N2H4 Sensing
3.4. Selectivity, Stability, and Repeatability
3.5. Detection of N2H4 in Real Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khushboo; Umar, A.; Kansal, S.K.; Mehta, S.K. Highly-sensitive and selective detection of hydrazine at gold electrode modified with PEG-coated CdS nanoparticles. Sens. Actuators B Chem. 2013, 188, 372–377. [Google Scholar] [CrossRef]
- Chen, S.; Hou, P.; Wang, J.; Liu, L.; Zhang, Q. A highly selective fluorescent probe based on coumarin for the imaging of N2H4 in living cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Mazloum-Ardakani, M.; Khoshroo, A.; Hosseinzadeh, L. Simultaneous determination of hydrazine and hydroxylamine based on fullerene-functionalized carbon nanotubes/ionic liquid nanocomposite. Sens. Actuators B Chem. 2015, 214, 132–137. [Google Scholar] [CrossRef]
- He, Y.P.; Zheng, J.B.; Dong, S.Y. Ultrasonic-electrodeposition of hierarchical flower-like cobalt on petalage-like graphene hybrid microstructures for hydrazine sensing. Analyst 2012, 137, 4814–4818. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, B.; Hamidi, H.; Bozorgzadeh, S. Sensitive and selective determination of hydrazine using glassy carbon electrode modified with Pd nanoparticles decorated multiwalled carbon nanotubes. Anal. Bioanal. Chem. 2010, 398, 1411–1416. [Google Scholar] [CrossRef]
- He, Y.P.; Zheng, J.B.; Sheng, Q.L. Cobalt nanoparticles as sacrificial templates for the electrodeposition of palladium nanomaterials in an ionic liquid, and its application to electrochemical sensing of hydrazine. Microchim. Acta 2012, 177, 479–484. [Google Scholar] [CrossRef]
- Koçak, S.; Aslışen, B. Hydrazine oxidation at gold nanoparticles and poly (bromocresol purple) carbon nanotube modified glassy carbon electrode. Sens. Actuators B Chem. 2014, 196, 610–618. [Google Scholar] [CrossRef]
- Do Nascimento, A.S.; Cabral, P.E.; Fontes, A.; Santos, B.S.; de Carvalho, F.R.; Stragevitch, L.; Leite, E.S. CdSe quantum dots as fluorescent nanomarkers for diesel oil. Fuel 2019, 239, 1055–1060. [Google Scholar] [CrossRef]
- Liu, M.P.; Liu, T.; Xiao, D. A FRET chemsensor based on graphene quantum dots for detecting and intracellular imaging of Hg2+. Talanta 2015, 143, 442–449. [Google Scholar] [CrossRef]
- Bogomolova, A.; Aldissi, M. Real-time aptamer quantum dot fluorescent flow sensor. Biosens. Bioelectron. 2011, 26, 4099–4103. [Google Scholar] [CrossRef]
- Li, X.C.; Zhao, Y.P.; Yin, J.L.; Lin, W.Y. Organic fluorescent probes for detecting mitochondrial membrane potential. Coord. Chem. Rev. 2020, 420, 213419. [Google Scholar] [CrossRef]
- Zhao, X.J.; Huang, C.Z. Small organic molecules as fluorescent probes for nucleotides and their derivatives. Trend. Anal. Chem. 2010, 29, 354–367. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Ke, W.Q.; Lou, H.Y.; Han, Y.H.; Wan, J.M. A novel fluorescent metal-organic framework based on porphyrin and AIE for ultra-high sensitivity and selectivity detection of Pb2+ ions in aqueous solution. Dyes Pigment. 2021, 196, 109802. [Google Scholar] [CrossRef]
- He, R.; Wang, Y.L.; Ma, H.F.; Yin, S.G.; Liu, Q.Y. Eu3+-functionalized metal-organic framework composite as ratiometric fluorescent sensor for highly selective detecting urinary 1-hydroxypyrene. Dyes Pigment. 2018, 151, 342–347. [Google Scholar] [CrossRef]
- Zhou, Z.D.; Wang, C.Y.; Zhu, G.S.; Du, B.; Yu, B.Y.; Wang, C.C. Water-stable europium(III) and terbium(III)-metal organic frameworks as fluorescent sensors to detect ions, antibiotics and pesticides in aqueous solutions. J. Mol. Struct. 2022, 1251, 132009. [Google Scholar] [CrossRef]
- Halawa, M.I.; Lai, J.; Xu, G. Gold nanoclusters: Synthetic strategies and recent advances in fluorescent sensing. Mater. Today Nano 2018, 3, 9–27. [Google Scholar] [CrossRef]
- Wang, C.X.; Wu, J.P.; Jiang, K.L.; Humphrey, M.G.; Zhang, C. Stable Ag nanoclusters-based nano-sensors: Rapid sonochemical synthesis and detecting Pb2+ in living cells. Sens. Actuators B Chem. 2017, 238, 1136–1143. [Google Scholar] [CrossRef]
- Li, S.X.; Chen, D.J.; Zheng, F.Y.; Zhou, H.F.; Jiang, S.X.; Wu, Y.J. Water-Soluble and Lowly Toxic Sulphur Quantum Dots. Adv. Funct. Mater. 2015, 24, 7133–7138. [Google Scholar] [CrossRef]
- Duan, Y.X.; Tan, J.S.; Huang, Z.M.; Deng, Q.M.; Liu, S.J.; Wang, G.; Li, L.G.; Zhou, L. Facile synthesis of carboxymethyl cellulose sulfur quantum dots for live cell imaging and sensitive detection of Cr(VI) and ascorbic acid. Carbohydr. Polym. 2020, 249, 116882. [Google Scholar] [CrossRef]
- Gao, P.X.; Wang, G.; Zhou, L. Luminescent Sulfur Quantum Dots: Synthesis, Properties and Potential Applications. ChemPhotoChem 2020, 4, 5235–5244. [Google Scholar] [CrossRef]
- Lei, J.H.; Huang, Z.M.; Gao, P.X.; Sun, J.H.; Zhou, L. Polyvinyl Alcohol Enhanced Fluorescent Sulfur Quantum Dots for Highly Sensitive Detection of Fe3+ and Temperature in Cells. Part. Part. Syst. Charact. 2021, 38, 2000332. [Google Scholar] [CrossRef]
- Li, X.Q.; Li, M.Q.; Chen, Y.Z.; Qiao, G.X.; Liu, Q.; Zhou, Z.; Liu, W.Q.; Wang, Q.M. Chemical sensing failed by aggregation-caused quenching? A case study enables liquid/solid two-phase determination of N2H4. Chem. Eng. J. 2021, 415, 128975. [Google Scholar] [CrossRef]
- Li, D.D.; Zhang, Y.P.; Fan, Z.Y.; Chen, J.; Yu, J.H. Coupling of chromophores with exactly opposite luminescence behaviours in mesostructured organosilicas for high-efficiency multicolour emission. Chem. Sci. 2015, 6, 6097–6101. [Google Scholar] [CrossRef] [Green Version]
- Li, D.H.; Liu, L.; Yang, H.G.; Ma, J.; Wang, H.L.; Pan, J.M. A novel dual-response triphenylamine-based fluorescence sensor for special detection of hydrazine in water, Mater. Sci. Eng. B Adv. 2022, 276, 115556. [Google Scholar] [CrossRef]
- Xie, Y.; Yan, L.Q.; Tang, Y.J.; Tang, M.H.; Wang, S.Y.; Bi, L.; Sun, W.Y.; Li, J.P. A Smart Fluorescent Probe Based on Salicylaldehyde Schiff’s Base with AIE and ESIPT Characteristics for the Detections of N2H4 and ClO−. J. Fluoresc. 2019, 29, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.Q.; Li, M.; Zhang, Y.Y.; Yin, Y.G.; Lin, W.Y. Engineering an AIE N2H4 fluorescent probe based on alpha-cyanostilbene derivative with large Stokes shift and its versatile applications in solution, solid-state and biological systems. Sens. Actuators B Chem. 2021, 329, 129232. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Wu, W.N.; Xu, Z.Q.; Xu, Z.H.; Zhao, X.L.; Fan, Y.C. A novel ‘turn-on’ coumarin-based fluorescence probe with aggregation-induced emission (AIE) for sensitive detection of hydrazine and its imaging in living cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 222, 117272. [Google Scholar] [CrossRef]
- Chen, Z.Z.; Deng, Y.H.; Zhang, T.; Dong, W.K. A novel bifunctional-group salamo-like multi-purpose dye probe based on ESIPT and RAHB effect: Distinction of cyanide and hydrazine through optical signal differential protocol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 262, 120084. [Google Scholar] [CrossRef]
- Li, X.; Yin, J.W.; Liu, W.Y.; Yang, Y.T.; Xu, W.Z.; Li, W. A Novel Double Fluorescence-Suppressed Probe for the Detection of Hydrazine. ChemistrySelect 2020, 4, 14069–14074. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, X.X.; Qu, W.B.; Shi, T.; Liu, H.; He, H.P.; Zhang, X.H.; Wang, S.F. A highly selective HBT-based “turn-on” fluorescent probe for hydrazine detection and its application. Tetrahedron Lett. 2017, 58, 2596–2601. [Google Scholar] [CrossRef]
- Jiang, X.Z.; Zhen, L.; Mingqin, S.G.; Yi, S.L.; Zeng, X.Y.; Zhang, Y.L.; Hou, L.X. A fluorescence “turn-on” sensor for detecting hydrazine in environment. Microchem. J. 2020, 152, 104376. [Google Scholar] [CrossRef]
- Das, A.K.; Goswami, S. 2-Hydroxy-1-naphthaldehyde: A versatile building block for the development of sensors in supramolecular chemistry and molecular recognition. Sens. Actuators B Chem. 2017, 245, 1062–1125. [Google Scholar] [CrossRef]
- Ping, Y.; David, R.W. Calculation for Fluorescence Modulation by Absorbing Species and Its Application to Measurements Using Optical Fibers. Anal. Chem. 1987, 59, 2391–2394. [Google Scholar]
- Wang, H.G.; Wang, Z.G.; Xiong, Y.; Kershaw, S.V.; Li, T.Z.; Wang, Y.; Zhai, Y.Q.; Rogach, A.L. Hydrogen Peroxide Assisted Synthesis of Highly Luminescent Sulfur Quantum Dots. Angew. Chem. Int. Ed. 2019, 58, 7040–7044. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Huang, W.; Liang, Y.; Yu, H.L. A low-cost and label-free assay for hydrazine using MnO2 nanosheets as colorimetric probes. Sens. Actuators B Chem. 2015, 220, 927–931. [Google Scholar] [CrossRef]
- He, L.Y.; Lu, Y.X.; Wang, F.Y.; Jing, W.J.; Chen, Y.; Liu, Y.Y. Colorimetric sensing of silver ions based on glutathione-mediated MnO2 nanosheets. Sens. Actuators B Chem. 2018, 254, 468–474. [Google Scholar] [CrossRef]
- Amjadi, M.; Hallaj, T.; Kouhi, Z. An enzyme-free fluorescent probe based on carbon dots-MnO2 nanosheets for determination of uric acid. J. Photochem. Photobiol. A Chem. 2018, 356, 603–609. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Li, Y.X.; Zhang, C.Y.; Zhang, Q.F.; Huang, X.A.; Yang, M.D.; Shahzad, S.A.; Lo, K.K.W.; Yu, C.; Jiang, S.C. Fluorescence turn-on detection of alkaline phosphatase activity based on controlled release of PEI-capped Cu nanoclusters from MnO2 nanosheets. Anal. Bioanal. Chem. 2017, 409, 4771–4778. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.F.; Yi, R.H.; Ai, S.F.; Cheng, H.R.; Jia, W.Z. Synthesis and Application of a Flavone-based Fluorescent Probe for Detection of Hydrazine. Chin. J. Anal. Chem. 2019, 46, 511–516. [Google Scholar]
- Jiang, Q.; Wang, Z.L.; Li, M.X.; Song, J.; Yang, Y.Q.; Xu, X.; Xu, H.J.; Wang, S.F. A novel dual-response fluorescent probe based on nopinone for discriminative detection of hydrazine and bisulfate from different emission channels. Tetrahedron Lett. 2020, 61, 152103. [Google Scholar] [CrossRef]







| Method | Linear Range (M) | Detection Limit (M) | Ref. |
|---|---|---|---|
| ZY8 a | 1.6 × 10−7–6.2 × 10−5 | 1.6 × 10−7 | [39] |
| PBAS b | 0–2 × 10−5 | 4.1 × 10−7 | [25] |
| CEFN c | 0–6 × 10−5 | 9.6 × 10−8 | [40] |
| HBTM d | 0–1.4 × 10−4 | 2.9 × 10−7 | [30] |
| SQDs@MnO2 NS | 10−7–10−2 | 7.2 × 10−8 | This work |
| Sample | Added (M) | Found (M) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 10−2 | 1.073 × 10−2 | 107.3 | 1.4 | |
| Lake water | 10−5 | 0.9021 × 10−5 | 90.21 | 2.1 |
| 10−7 | 0.9624 × 10−7 | 96.24 | 1.1 | |
| 10−2 | 1.091 × 10−2 | 109.1 | 2.2 | |
| River water | 10−5 | 1.032 × 10−5 | 103.2 | 0.9 |
| 10−7 | 0.9254 × 10−7 | 92.54 | 1.7 | |
| 10−2 | 0.9691 × 10−2 | 96.91 | 4.5 | |
| Serum | 10−5 | 0.9967 × 10−5 | 99.67 | 1.9 |
| 10−7 | 1.027 × 10−7 | 102.7 | 2.8 | |
| 10−2 | 0.9851 × 10−2 | 98.51 | 3.1 | |
| Saliva | 10−5 | 0.9741 × 10−5 | 97.41 | 1.6 |
| 10−7 | 1.016 × 10−7 | 101.6 | 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, X.; Guo, W.; Luan, F.; Tian, C.; Zhuang, X.; Zhao, L. A Novel Turn-On Fluorescent Sensor Based on Sulfur Quantum Dots and MnO2 Nanosheet Architectures for Detection of Hydrazine. Nanomaterials 2022, 12, 2207. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12132207
Li X, Wang X, Guo W, Luan F, Tian C, Zhuang X, Zhao L. A Novel Turn-On Fluorescent Sensor Based on Sulfur Quantum Dots and MnO2 Nanosheet Architectures for Detection of Hydrazine. Nanomaterials. 2022; 12(13):2207. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12132207
Chicago/Turabian StyleLi, Xin, Xiaobin Wang, Wei Guo, Feng Luan, Chunyuan Tian, Xuming Zhuang, and Lijun Zhao. 2022. "A Novel Turn-On Fluorescent Sensor Based on Sulfur Quantum Dots and MnO2 Nanosheet Architectures for Detection of Hydrazine" Nanomaterials 12, no. 13: 2207. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12132207