Surface Chelation Enabled by Polymer-Doping for Self-Healable Perovskite Solar Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of PAT Polymer
2.3. Fabrication of PSC Devices
2.4. Solar Cells Characterizations
2.5. General Characterizations
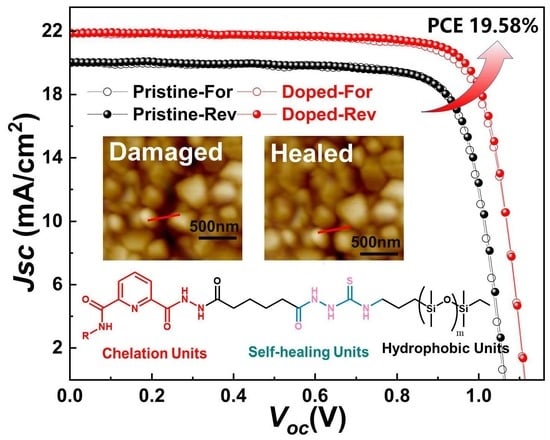
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grancini, G.; Nazeeruddin, M.K. Dimensional tailoring of hybrid perovskites for photovoltaics. Nat. Rev. Mater. 2018, 4, 4–22. [Google Scholar] [CrossRef]
- Luo, D.; Su, R.; Zhang, W.; Gong, Q.; Zhu, R. Minimizing non-radiative recombination losses in perovskite solar cells. Nat. Rev. Mater. 2019, 5, 44–60. [Google Scholar] [CrossRef]
- Leijtens, T.; Bush, K.A.; Prasanna, R.; McGehee, M.D. Opportunities and challenges for tandem solar cells using metal halide perovskite semiconductors. Nat. Energy 2018, 3, 828–838. [Google Scholar] [CrossRef]
- Meng, X.; Cai, Z.; Zhang, Y.; Hu, X.; Xing, Z.; Huang, Z.; Huang, Z.; Cui, Y.; Hu, T.; Su, M.; et al. Chen, Bio-inspired vertebral design for scalable and flexible perovskite solar cells. Nat. Commun. 2020, 11, 3016. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Meng, X.; Yang, X.; Huang, Z.; Xing, Z.; Li, P.; Tan, L.; Su, M.; Li, F.; Chen, Y.; et al. Cementitious grain-boundary passivation for flexible perovskite solar cells with superior environmental stability and mechanical robustness. Sci. Bull. 2021, 66, 527–535. [Google Scholar] [CrossRef]
- Lim, K.-G.; Han, T.-H.; Lee, T.-W. Engineering electrodes and metal halide perovskite materials for flexible/stretchable perovskite solar cells and light-emitting diodes. Energy Environ. Sci. 2021, 14, 2009–2035. [Google Scholar] [CrossRef]
- Hui, W.; Chao, L.; Lu, H.; Xia, F.; Wei, Q.; Su, Z.; Niu, T.; Tao, L.; Du, B.; Li, D.; et al. Stabilizing black-phase formamidinium perovskite formation at room temperature and high humidity. Science 2021, 371, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Bi, D.; Yi, C.; Luo, J.; Décoppet, J.-D.; Zhang, F.; Zakeeruddin, S.M.; Li, X.; Hagfeldt, A.; Grätzel, M. Polymer-templated nucleation and crystal growth of perovskite films for solar cells with efficiency greater than 21%. Nat. Energy 2016, 1, 16142. [Google Scholar] [CrossRef]
- YKim, Y.Y.; Yang, T.-Y.; Suhonen, R.; Kemppainen, A.; Hwang, K.; Jeon, N.J.; Seo, J. Roll-to-roll gravure-printed flexible perovskite solar cells using eco-friendly antisolvent bathing with wide processing window. Nat. Commun. 2020, 11, 5146. [Google Scholar]
- Dong, Q.; Chen, M.; Liu, Y.; Eickemeyer, F.T.; Zhao, W.; Dai, Z.; Yin, Y.; Jiang, C.; Feng, J.; Jin, S.; et al. Flexible perovskite solar cells with simultaneously improved efficiency, operational stability, and mechanical reliability. Joule 2021, 5, 1587–1601. [Google Scholar] [CrossRef]
- Collavini, S.; Cabrera-Espinoza, A.; Delgado, J.L. Organic Polymers as Additives in Perovskite Solar Cells. Macromolecules 2021, 54, 5451–5463. [Google Scholar] [CrossRef]
- Boyd, C.C.; Cheacharoen, R.; Leijtens, T.; McGehee, M.D. Understanding Degradation Mechanisms and Improving Stability of Perovskite Photovoltaics. Chem. Rev. 2019, 119, 3418–3451. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Xing, Z.; Hu, X.; Huang, Z.; Hu, T.; Tan, L.; Li, F.; Chen, Y. Stretchable Perovskite Solar Cells with Recoverable Performance. Angew. Chem. Int. Ed. Engl. 2020, 59, 16602–16608. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Meng, X.; Xiao, S.; Xing, Z.; Fu, Q.; Wang, H.; Gong, C.; Hu, T.; Hu, X.; Guo, R.; et al. Wearable Tin-Based Perovskite Solar Cells Achieved by a Crystallographic Size Effect. Angew. Chem. Int. Ed. Engl. 2021, 60, 14693–14700. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, S.; Lou, Y.; Liu, Q.; Li, M.; Okada, H.; Wang, Z. Perovskite Films with Reduced Interfacial Strains via a Molecular-Level Flexible Interlayer for Photovoltaic Application. Adv. Mater. 2020, 32, e2001479. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, P.; Wang, M.; Huang, S.; Zhao, Z.; Tan, S.; Han, T.; Lee, J.; Huang, T.; Wang, R.; et al. A Polymerization-Assisted Grain Growth Strategy for Efficient and Stable Perovskite Solar Cells. Adv. Mater. 2020, 32, e1907769. [Google Scholar] [CrossRef]
- TNiu, T.; Lu, J.; Munir, R.; Li, J.; Barrit, D.; Zhang, X.; Hu, H.; Yang, Z.; Amassian, A.; Zhao, K.; et al. Stable High-Performance Perovskite Solar Cells via Grain Boundary Passivation. Adv. Mater. 2018, 30, e1706576. [Google Scholar]
- Duan, X.; Li, X.; Tan, L.; Huang, Z.; Yang, J.; Liu, G.; Lin, Z.; Chen, Y. Controlling Crystal Growth via an Autonomously Longitudinal Scaffold for Planar Perovskite Solar Cells. Adv. Mater. 2020, 32, e2000617. [Google Scholar] [CrossRef]
- Yoon, J.; Sung, H.; Lee, G.; Cho, W.; Ahn, N.; Jung, H.S.; Choi, M. Superflexible, high-efficiency perovskite solar cells utilizing graphene electrodes: Towards future foldable power sources. Energy Environ. Sci. 2017, 10, 337–345. [Google Scholar] [CrossRef]
- Hu, X.; Huang, Z.; Li, F.; Su, M.; Huang, Z.; Zhao, Z.; Cai, Z.; Yang, X.; Meng, X.; Li, P.; et al. Nacre-inspired crystallization and elastic “brick-and-mortar” structure for a wearable perovskite solar module. Energy Environ. Sci. 2019, 12, 979–987. [Google Scholar] [CrossRef]
- Hu, X.; Meng, X.; Zhang, L.; Zhang, Y.; Cai, Z.; Huang, Z.; Su, M.; Wang, Y.; Li, M.; Li, F.; et al. A Mechanically Robust Conducting Polymer Network Electrode for Efficient Flexible Perovskite Solar Cells. Joule 2019, 3, 2205–2218. [Google Scholar] [CrossRef]
- Zhou, Y.; Khan, T.M.; Liu, J.-C.; Fuentes-Hernandez, C.; Shim, J.W.; Najafabadi, E.; Youngblood, J.; Moon, R.; Kippelen, B. Efficient recyclable organic solar cells on cellulose nanocrystal substrates with a conducting polymer top electrode deposited by film-transfer lamination. Org. Electron. 2014, 15, 661–666. [Google Scholar] [CrossRef]
- Zhou, H.; Park, J.; Lee, Y.; Park, J.; Kim, J.; Kim, J.S.; Lee, H.; Jo, S.H.; Cai, X.; Li, L.; et al. Water Passivation of Perovskite Nanocrystals Enables Air-Stable Intrinsically Stretchable Color-Conversion Layers for Stretchable Displays. Adv. Mater. 2020, 32, e2001989. [Google Scholar] [CrossRef] [PubMed]
- Zuo, C.; Vak, D.; Angmo, D.; Ding, L.; Gao, M. One-step roll-to-roll air processed high efficiency perovskite solar cells. Nano Energy 2018, 46, 185–192. [Google Scholar] [CrossRef]
- Yang, D.; Yang, R.; Priya, S.; Liu, F. Recent Advances in Flexible Perovskite Solar Cells: Fabrication and Applications. Angew. Chem. Int. Ed. Engl. 2019, 58, 4466–4483. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Li, H.; Yan, Y.; Chi, B.; Felice, K.M.; Moore, R.B.; Magill, B.A.; Mudiyanselage, R.R.H.H.; Khodaparast, G.A.; Sanghadasa, M.; et al. Highly-Stable Organo-Lead Halide Perovskites Synthesized Through Green Self-Assembly Process. Sol. RRL 2018, 2, 1800052. [Google Scholar] [CrossRef]
- Wen, L.; Rao, Y.; Zhu, M.; Li, R.; Zhan, J.; Zhang, L.; Wang, L.; Li, M.; Pang, S.; Zhou, Z. Reducing Defects Density and Enhancing Hole Extraction for Efficient Perovskite Solar Cells Enabled by pi-Pb(2+) Interactions. Angew. Chem. Int. Ed. Engl. 2021, 60, 17356–17361. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, X.; Chen, J.; Xiong, T.; Jiang, B.; Huang, Y. Self-healing and stretchable PDMS-based bifunctional sensor enabled by synergistic dynamic interactions. Chem. Eng. J. 2021, 412, 128734. [Google Scholar] [CrossRef]
- Na Quan, L.; Rand, B.P.; Friend, R.H.; Mhaisalkar, S.G.; Lee, T.-W.; Sargent, E.H. Perovskites for Next-Generation Optical Sources. Chem. Rev. 2019, 119, 7444–7477. [Google Scholar] [CrossRef]
- Di Giacomo, F.; Fakharuddin, A.; Jose, R.; Brown, T.M. Progress, challenges and perspectives in flexible perovskite solar cells. Energy Environ. Sci. 2016, 9, 3007–3035. [Google Scholar] [CrossRef]
- Shu, L.; Ke, S.; Fei, L.; Huang, W.; Wang, Z.; Gong, J.; Jiang, X.; Wang, L.; Li, F.; Lei, S.; et al. Photoflexoelectric effect in halide perovskites. Nat. Mater. 2020, 19, 605–609. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Shi, X.; Wu, G.; Huang, Y. Surface Chelation Enabled by Polymer-Doping for Self-Healable Perovskite Solar Cells. Nanomaterials 2022, 12, 3125. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12183125
Zhang K, Shi X, Wu G, Huang Y. Surface Chelation Enabled by Polymer-Doping for Self-Healable Perovskite Solar Cells. Nanomaterials. 2022; 12(18):3125. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12183125
Chicago/Turabian StyleZhang, Kuiyuan, Xiangrong Shi, Guangyu Wu, and Yudong Huang. 2022. "Surface Chelation Enabled by Polymer-Doping for Self-Healable Perovskite Solar Cells" Nanomaterials 12, no. 18: 3125. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12183125