Hierarchical Co–Pi Clusters/Fe2O3 Nanorods/FTO Micropillars 3D Branched Photoanode for High-Performance Photoelectrochemical Water Splitting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Periodic FTO Micropillars
2.2. Synthesis of Hematite Nanorods
2.3. Characterization
2.4. PEC Measurements
3. Results
3.1. Characterization of the Fabricated FTO-M
3.2. Characterization of the Fabricated Fe2O3/Co–Pi Photoanode
3.3. Optical Properties of the Hematite Nanorod on the Micropillar FTO Photoanode
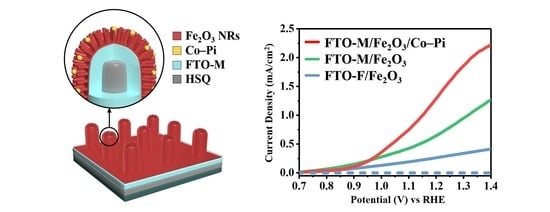
3.4. PEC Performance of the Micropillar-Patterned Hematite Nanorod Photoanode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Reece, S.Y.; Hamel, J.A.; Sung, K.; Jarvi, T.D.; Esswein, A.J.; Pijpers, J.J.H.; Nocera, D.G. Wireless solar water splitting using silicon-based semiconductors and earth-abundant catalysts. Science 2011, 334, 645–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landman, A.; Dotan, H.; Shter, G.E.; Wullenkord, M.; Houaijia, A.; Maljusch, A.; Grader, G.S.; Rothschild, A. Photoelectrochemical water splitting in separate oxygen and hydrogen cells. Nat. Mater. 2017, 16, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Yang, X.; Mayer, M.T.; Hoyt, H.; Xie, J.; McMahon, G.; Bischoping, G.; Wang, D. Hematite-based water splitting with low turn-on voltages. Angew. Chem. 2013, 52, 12924–12927. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Youn, D.H.; Kang, K.; Lee, J.S. Highly conformal deposition of an ultrathin FeOOH layer on a hematite nanostructure for efficient solar water splitting. Angew. Chem. 2016, 128, 11012–11016. [Google Scholar] [CrossRef]
- Wang, W.; Jin, C.; Qi, L. Hierarchical CdS nanorod@SnO2 nanobowl arrays for efficient and stable photoelectrochemical hydrogen generation. Small 2018, 14, e1801352. [Google Scholar] [CrossRef]
- Pan, Q.; Zhang, H.; Yang, Y.; Cheng, C. 3D Brochosomes-like TiO2/WO3/BiVO4 arrays as photoanode for photoelectrochemical hydrogen production. Small 2019, 15, e1900924. [Google Scholar] [CrossRef]
- Quang, N.D.; Hu, W.; Chang, H.S.; Van, P.C.; Viet, D.D.; Jeong, J.R.; Seo, D.B.; Kim, E.T.; Kim, C.; Kim, D. Fe2O3 hierarchical tubular structure decorated with cobalt phosphide (CoP) nanoparticles for efficient photoelectrochemical water splitting. Chem. Eng. J. 2021, 417, 129278. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, H.; Tu, W.; Zhu, X.; Yao, Y.; Zhou, Y.; Zou, Z. Host/guest nanostructured photoanodes integrated with targeted enhancement strategies for photoelectrochemical water splitting. Adv. Sci. (Weinh) 2022, 9, e2103744. [Google Scholar] [CrossRef]
- Wang, L.; Palacios-Padrós, A.; Kirchgeorg, R.; Tighineanu, A.; Schmuki, P. Enhanced photoelectrochemical water splitting efficiency of a hematite-ordered Sb:SnO2 host-guest system. ChemSusChem 2014, 7, 421–424. [Google Scholar] [CrossRef]
- Resasco, J.; Zhang, H.; Kornienko, N.; Becknell, N.; Lee, H.; Guo, J.; Briseno, A.L.; Yang, P. TiO2/BiVO4 nanowire heterostructure photoanodes based on type II band alignment. ACS Cent. Sci. 2016, 2, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Zhao, C.; Giri, B.; Allen, P.; Xu, X.; Joshi, H.; Fan, Y.; Titova, L.V.; Rao, P.M. High light absorption and charge separation efficiency at low applied voltage from Sb-doped SnO2/BiVO4 core/shell nanorod-array photoanodes. Nano Lett. 2016, 16, 3463–3474. [Google Scholar] [CrossRef]
- Garcia-Torregrosa, I.; Wijten, J.H.J.; Zanoni, S.; Oropeza, F.E.; Hofmann, J.P.; Hensen, E.J.M.; Weckhuysen, B.M. Template-free nanostructured fluorine-doped tin oxide scaffolds for photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2019, 11, 36485–36496. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Nguyen, T.D.; Yeo, L.P.; Tan, C.K.; Gan, L.; Tok, A.I.Y. Periodic FTO IOs/CdS NRs/CdSe clusters with superior light scattering ability for improved photoelectrochemical performance. Small 2020, 16, e1905826. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, X.; Ling, H.; Tan, C.K.; Yeo, L.P.; Grimsdale, A.C.; Tok, A.I.Y. 3D FTO/FTO-nanocrystal/TiO2 composite inverse opal photoanode for efficient photoelectrochemical water splitting. Small 2018, 14, e1800395. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qiu, Y.; Wei, Z.; Lin, Q.; Zhang, Q.; Yan, K.; Chen, H.; Xiao, S.; Fan, Z.; Yang, S. A three-dimensional hexagonal fluorine-doped tin oxide nanocone array: A superior light harvesting electrode for high performance photoelectrochemical water splitting. Energy Environ. Sci. 2014, 7, 3651–3658. [Google Scholar] [CrossRef]
- Ju, S.; Kang, H.; Jun, J.; Son, S.; Park, J.; Kim, W.; Lee, H. Periodic micropillar-patterned FTO/BiVO4 with superior light absorption and separation efficiency for efficient PEC performance. Small 2021, 17, e2006558. [Google Scholar] [CrossRef]
- Ju, S.; Seok, H.J.; Jun, J.; Huh, D.; Son, S.; Kim, K.; Kim, W.; Baek, S.; Kim, H.K.; Lee, H. Fully blossomed WO3/BiVO4 structure obtained via active facet engineering of patterned FTO for highly efficient water splitting. Appl. Catal. B 2020, 263, 118362. [Google Scholar] [CrossRef]
- Jeon, T.H.; Moon, G.H.; Park, H.; Choi, W. Ultra-efficient and durable photoelectrochemical water oxidation using elaborately designed hematite nanorod arrays. Nano Energy 2017, 39, 211–218. [Google Scholar] [CrossRef]
- Jeon, T.H.; Choi, W.; Park, H. Cobalt-phosphate complexes catalyze the photoelectrochemical water oxidation of BiVO4 electrodes. Phys. Chem. Chem. Phys. 2011, 13, 21392–21401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, X.; Zhang, H.; Pan, Q.; Bian, J.; Chen, Z.; Cheng, C. 3D ordered urchin-like TiO2@Fe2O3 arrays photoanode for efficient photoelectrochemical water splitting. Appl. Surf. Sci. 2019, 470, 668–676. [Google Scholar] [CrossRef]
- Liu, C.; Wang, F.; Zhang, J.; Wang, K.; Qiu, Y.; Liang, Q.; Chen, Z. Efficient photoelectrochemical water splitting by g-C3N4/TiO2 nanotube array heterostructures. Nano Micro Lett. 2018, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Xie, J.; Guo, C.; Li, C.M. Modification of a thin layer of α-Fe2O3 onto a largely voided TiO2 nanorod array as a photoanode to significantly improve the photoelectrochemical performance toward water oxidation. RSC Adv. 2015, 5, 62611–62618. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, T.; Jiang, K.; Da, P.; Peng, Z.; Tang, J.; Kong, B.; Cai, W.B.; Yang, Z.; Zheng, G. Reduced mesoporous Co3O4 nanowires as efficient water oxidation electrocatalysts and supercapacitor electrodes. Adv. Energy Mater. 2014, 4, 1400696. [Google Scholar] [CrossRef]
- Ai, G.; Mo, R.; Li, H.; Zhong, J. Cobalt phosphate modified TiO2 nanowire arrays as co-catalysts for solar water splitting. Nanoscale 2015, 7, 6722–6728. [Google Scholar] [CrossRef] [PubMed]
- McDonald, K.J.; Choi, K.S. Photodeposition of co-based oxygen evolution catalysts on α-Fe2O3 photoanodes. Chem. Mater. 2011, 23, 1686–1693. [Google Scholar] [CrossRef]
- Ai, L.; Niu, Z.; Jiang, J. Mechanistic insight into oxygen evolution electrocatalysis of surface phosphate modified cobalt phosphide nanorod bundles and their superior performance for overall water splitting. Electrochim. Acta 2017, 242, 355–363. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, D.; Wang, S.; Liang, J.; Qin, L.; Sun, X.; Huang, Y. Photoassisted electrodeposition of cobalt-phosphate cocatalyst on BiFeO3 thin film photoanode for highly efficient photoelectrochemical performances of water oxidation. J. Electrochem. Soc. 2019, 166, D308–D314. [Google Scholar] [CrossRef]
- Raut, H.K.; Ganesh, V.A.; Nair, A.S.; Ramakrishna, S. Anti-reflective coatings: A critical, in-depth review. Energy Environ. Sci. 2011, 4, 3779–3804. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, J.; Chen, S.; Bai, J.; Li, J.; Zhang, Y.; Li, L.; Xia, L.; Rahim, M.; Xu, Q.; et al. Bird-nest structured ZnO/TiO2 as a direct Z-scheme photoanode with enhanced light harvesting and carriers kinetics for highly efficient and stable photoelectrochemical water splitting. Appl. Catal. B 2020, 267, 118599. [Google Scholar] [CrossRef]
- Dotan, H.; Sivula, K.; Grätzel, M.; Rothschild, A.; Warren, S.C. Probing the photoelectrochemical properties of hematite (α-Fe2O3) electrodes using hydrogen peroxide as a hole scavenger. Energy Environ. Sci. 2011, 4, 958–964. [Google Scholar] [CrossRef]
- Nair, V.; Perkins, C.L.; Lin, Q.; Law, M. Textured nanoporous Mo:BiVO4 photoanodes with high charge transport and charge transfer quantum efficiencies for oxygen evolution. Energy Environ. Sci. 2016, 9, 1412–1429. [Google Scholar] [CrossRef] [Green Version]
- Eftekharinia, B.; Moshaii, A.; Dabirian, A.; Vayghan, N.S. Optimization of charge transport in a Co-Pi modified hematite thin film produced by scalable electron beam evaporation for photoelectrochemical water oxidation. J. Mater. Chem. A 2017, 5, 3412–3424. [Google Scholar] [CrossRef]
- Li, L.; Liu, C.; Zhang, H.; Liang, P.; Mitsuzaki, N.; Chen, Z. Synergistic effect of Ti(OBu)4 and annealing regime on the structure, morphology and photoelectrochemical response of α-Fe2O3 photoanode. Electrochim. Acta 2018, 281, 246–256. [Google Scholar] [CrossRef]
- Yi, S.S.; Wulan, B.R.; Yan, J.M.; Jiang, Q. Highly efficient photoelectrochemical water splitting: Surface modification of cobalt-phosphate-loaded Co3O4/Fe2O3 p–n heterojunction nanorod arrays. Adv. Funct. Mater. 2019, 29, 1801902. [Google Scholar] [CrossRef]
- Bu, X.; Gao, Y.; Zhang, S.; Tian, Y. Amorphous cerium phosphate on P-doped Fe2O3 nanosheets for efficient photoelectrochemical water oxidation. Chem. Eng. J. 2019, 355, 910–919. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Z.; Guo, Z.; Ruan, M.; Yan, W. 3D branched Ca-Fe2O3/Fe2O3 decorated with Pt and co-pi: Improved charge-separation dynamics and photoelectrochemical performance. ChemSusChem 2019, 12, 3286–3295. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.; Ju, S.; Ha, J.; Choi, H.; Sung, H.; Lee, H. Hierarchical Co–Pi Clusters/Fe2O3 Nanorods/FTO Micropillars 3D Branched Photoanode for High-Performance Photoelectrochemical Water Splitting. Nanomaterials 2022, 12, 3664. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12203664
Kim N, Ju S, Ha J, Choi H, Sung H, Lee H. Hierarchical Co–Pi Clusters/Fe2O3 Nanorods/FTO Micropillars 3D Branched Photoanode for High-Performance Photoelectrochemical Water Splitting. Nanomaterials. 2022; 12(20):3664. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12203664
Chicago/Turabian StyleKim, Nakhyun, Sucheol Ju, Jisung Ha, Hojung Choi, Hansang Sung, and Heon Lee. 2022. "Hierarchical Co–Pi Clusters/Fe2O3 Nanorods/FTO Micropillars 3D Branched Photoanode for High-Performance Photoelectrochemical Water Splitting" Nanomaterials 12, no. 20: 3664. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12203664