Nano TiO2 and Molybdenum/Tungsten Iodide Octahedral Clusters: Synergism in UV/Visible-Light Driven Degradation of Organic Pollutants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Synthesis of Pure TiO2
2.4. Synthesis of nx@TiO2 (n = 1 (Mo) or 2 (W))
2.5. Photocatalytic Experiment
2.6. Cyclic Experiments
2.7. Photodegradation of RhB under Solar Light
2.8. Scavengers
3. Results and Discussion
3.1. Synthesis of Pure TiO2 and nx@TiO2 (n = 1 (Mo) or 2 (W))
3.2. Morphology and Composition
3.3. X-ray Photoelectron Spectroscopy (XPS)
3.4. Surface Area and Porous Structure
3.5. Absorption and Emission
3.6. Photocatalytic Degradation of RhB
3.7. Scavengers
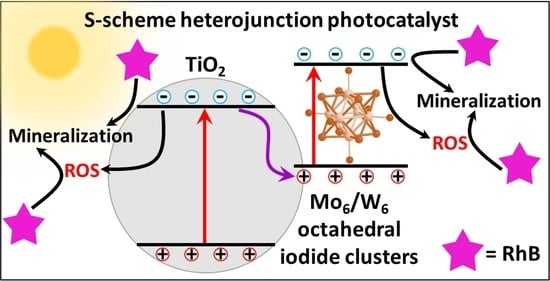
3.8. Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Environment Programme. Towards a Pollution-Free Planet: Background Report; United Nations: San Francisco, CA, USA, 2017. [Google Scholar]
- Gavrilescu, M. Water, Soil, and Plants Interactions in a Threatened Environment. Water 2021, 13, 2746. [Google Scholar] [CrossRef]
- UN Water. The United Nations World Water Development Report 2021: Valuing Water; UN Water: Geneva, Switzerland, 2021. [Google Scholar]
- Liu, Q. Pollution and Treatment of Dye Waste-Water. IOP Conf. Ser. Earth Environ. Sci. 2020, 514, 052001. [Google Scholar] [CrossRef]
- Ismail, M.; Akhtar, K.; Khan, M.I.; Kamal, T.; Khan, A.M.; Asiri, A.M.; Seo, J.; Khan, B.S. Pollution, Toxicity and Carcinogenicity of Organic Dyes and their Catalytic Bio-Remediation. Curr. Pharm. Des. 2019, 25, 3645–3663. [Google Scholar] [CrossRef] [PubMed]
- Shanker, U.; Rani, M.; Jassal, V. Degradation of Hazardous Organic Dyes in Water by Nanomaterials. Environ. Chem. Lett. 2017, 15, 623–642. [Google Scholar] [CrossRef]
- Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Cogliano, V. Carcinogenicity of Some Aromatic Amines, Organic Dyes, and Related Exposures. Lancet Oncol. 2008, 9, 322–323. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of Textile Dyes on Health and the Environment and Bioremediation Potential of Living Organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Sathya, K.; Nagarajan, K.; Carlin Geor Malar, G.; Rajalakshmi, S.; Raja Lakshmi, P. A Comprehensive Review on Comparison among Effluent Treatment Methods and Modern Methods of Treatment of Industrial Wastewater Effluent from Different Sources. Appl. Water Sci. 2022, 12, 70. [Google Scholar] [CrossRef]
- Akerdi, A.G.; Bahrami, S.H. Application of Heterogeneous Nano-Semiconductors for Photocatalytic Advanced Oxidation of Organic Compounds: A Review. J. Environ. Chem. Eng. 2019, 7, 103283. [Google Scholar] [CrossRef]
- Zhang, L.; Ran, J.; Qiao, S.-Z.; Jaroniec, M. Characterization of Semiconductor Photocatalysts. Chem. Soc. Rev. 2019, 48, 5184–5206. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, M.; Xu, J.; Li, C.; Yan, J.; Zhou, G.; Zhong, W.; Hao, T.; Song, J.; Xue, X.; et al. Single-Junction Organic Solar Cells with over 19% Efficiency Enabled by a Refined Double-Fibril Network Morphology. Nat. Mater. 2022, 21, 656–663. [Google Scholar] [CrossRef]
- Gao, W.; Qi, F.; Peng, Z.; Lin, F.R.; Jiang, K.; Zhong, C.; Kaminsky, W.; Guan, Z.; Lee, C.-S.; Marks, T.J.; et al. Achieving 19% Power Conversion Efficiency in Planar-Mixed Heterojunction Organic Solar Cells Using a Pseudosymmetric Electron Acceptor. Adv. Mater. 2022, 34, 2202089. [Google Scholar] [CrossRef]
- Ma, R.; Yan, C.; Yu, J.; Liu, T.; Liu, H.; Li, Y.; Chen, J.; Luo, Z.; Tang, B.; Lu, X.; et al. High-Efficiency Ternary Organic Solar Cells with a Good Figure-of-Merit Enabled by Two Low-Cost Donor Polymers. ACS Energy Lett. 2022, 7, 2547–2556. [Google Scholar] [CrossRef]
- Ma, D.S.; Yi, H.; Lai, C.; Liu, X.G.; Huo, X.Q.; An, Z.W.; Li, L.; Fu, Y.K.; Li, B.S.; Zhang, M.M.; et al. Critical Review of Advanced Oxidation Processes in Organic Wastewater Treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.L.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Thambiliyagodage, C. Activity Enhanced TiO2 Nanomaterials for Photodegradation of Dyes—A Review. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100592. [Google Scholar] [CrossRef]
- Haghighatmamaghani, A.; Haghighat, F.; Lee, C.S. Performance of Various Commercial TiO2 in Photocatalytic Degradation of a Mixture of Indoor Air Pollutants: Effect of Photocatalyst and Operating Parameters. Sci. Technol. Built Environ. 2019, 25, 600–614. [Google Scholar] [CrossRef]
- Piątkowska, A.; Janus, M.; Szymański, K.; Mozia, S. C-, N- and S-Doped TiO2 Photocatalysts: A Review. Catalysts 2021, 11, 144. [Google Scholar] [CrossRef]
- Li, K.; Teng, C.; Wang, S.; Min, Q. Recent Advances in TiO2-Based Heterojunctions for Photocatalytic CO2 Reduction With Water Oxidation: A Review. Front. Chem. 2021, 9, 637501. [Google Scholar] [CrossRef]
- Wei, L.; Yu, C.; Zhang, Q.; Liu, H.; Wang, Y. TiO2-Based Heterojunction Photocatalysts for Photocatalytic Reduction of CO2 into Solar Fuels. J. Mater. Chem. A 2018, 6, 22411–22436. [Google Scholar] [CrossRef]
- Barras, A.; Cordier, S.; Boukherroub, R. Fast Photocatalytic Degradation of Rhodamine B over [Mo6Br8(N3)6]2− Cluster Units under Sun Light Irradiation. Appl. Catal. B Environ. 2012, 123–124, 1–8. [Google Scholar] [CrossRef]
- Barras, A.; Das, M.R.; Devarapalli, R.R.; Shelke, M.V.; Cordier, S.; Szunerits, S.; Boukherroub, R. One-Pot Synthesis of Gold Nanoparticle/Molybdenum Cluster/Graphene Oxide Nanocomposite and Its Photocatalytic Activity. Appl. Catal. B Environ. 2013, 130–131, 270–276. [Google Scholar] [CrossRef]
- Kumar, S.; Khatri, O.P.; Cordier, S.; Boukherroub, R.; Jain, S.L. Graphene Oxide Supported Molybdenum Cluster: First Heterogenized Homogeneous Catalyst for the Synthesis of Dimethylcarbonate from CO2 and Methanol. Chem. Eur. J. 2015, 21, 3488–3494. [Google Scholar] [CrossRef] [PubMed]
- Feliz, M.; Atienzar, P.; Amela-Cortés, M.; Dumait, N.; Lemoine, P.; Molard, Y.; Cordier, S. Supramolecular Anchoring of Octahedral Molybdenum Clusters onto Graphene and Their Synergies in Photocatalytic Water Reduction. Inorg. Chem. 2019, 58, 15443–15454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanova, M.N.; Vorotnikov, Y.A.; Plotnikova, E.E.; Marchuk, M.V.; Ivanov, A.A.; Asanov, I.P.; Tsygankova, A.R.; Grayfer, E.D.; Fedorov, V.E.; Shestopalov, M.A. Hexamolybdenum Clusters Supported on Exfoliated h-BN Nanosheets for Photocatalytic Water Purification. Inorg. Chem. 2020, 59, 6439–6448. [Google Scholar] [CrossRef]
- Svezhentseva, E.V.; Vorotnikov, Y.A.; Solovieva, A.O.; Pozmogova, T.N.; Eltsov, I.V.; Ivanov, A.A.; Evtushok, D.V.; Miroshnichenko, S.M.; Yanshole, V.V.; Eling, C.J.; et al. From Photoinduced to Dark Cytotoxicity through an Octahedral Cluster Hydrolysis. Chem. Eur. J. 2018, 24, 17915–17920. [Google Scholar] [CrossRef]
- Ding, X.-Z.; Qi, Z.-Z.; He, Y.-Z. Effect of Hydrolysis Water on the Preparation of Nano-Crystalline Titania Powders via a Sol-Gel Process. J. Mater. Sci. Lett. 1995, 14, 21–22. [Google Scholar] [CrossRef]
- Wang, C.-C.; Ying, J.Y. Sol−Gel Synthesis and Hydrothermal Processing of Anatase and Rutile Titania Nanocrystals. Chem. Mater. 1999, 11, 3113–3120. [Google Scholar] [CrossRef]
- May-Lozano, M.; Ramos-Reyes, G.M.; López-Medina, R.; Martínez-Delgadillo, S.A.; Flores-Moreno, J.; Hernández-Pérez, I. Effect of the Amount of Water in the Synthesis of B-TiO2: Orange II Photodegradation. Int. J. Photochem. 2014, 2014, 721216. [Google Scholar] [CrossRef] [Green Version]
- Pronina, E.V.; Pozmogova, T.N.; Vorotnikov, Y.A.; Ivanov, A.A.; Shestopalov, M.A. The Role of Hydrolysis in Biological Effects of Molybdenum Cluster with DMSO Ligands. J. Biol. Inorg. Chem. 2022, 27, 111–119. [Google Scholar] [CrossRef]
- Kumar, A.; Mondal, S.; Rao, K.S.R.K. Critical Investigation of High Performance Spin-Coated High-Kappa Titania Thin Films Based MOS Capacitor. J. Mater. Sci. Mater. Electron. 2016, 27, 5264–5270. [Google Scholar] [CrossRef]
- Nawaz, R.; Kait, C.F.; Chia, H.Y.; Isa, M.H.; Huei, L.W. Glycerol-Mediated Facile Synthesis of Colored Titania Nanoparticles for Visible Light Photodegradation of Phenolic Compounds. Nanomaterials 2019, 9, 1586. [Google Scholar] [CrossRef] [Green Version]
- Perron, H.; Vandenborre, J.; Domain, C.; Drot, R.; Roques, J.; Simoni, E.; Ehrhardt, J.J.; Catalette, H. Combined Investigation of Water Sorption on TiO2 rutile (110) Single Crystal Face: XPS vs. Periodic DFT. Surf. Sci. 2007, 601, 518–527. [Google Scholar] [CrossRef] [Green Version]
- Benkoula, S.; Sublemontier, O.; Patanen, M.; Nicolas, C.; Sirotti, F.; Naitabdi, A.; Gaie-Levrel, F.; Antonsson, E.; Aureau, D.; Ouf, F.X.; et al. Water Adsorption on TiO2 Surfaces Probed by Soft X-Ray Spectroscopies: Bulk Materials vs. Isolated Nanoparticles. Sci. Rep. 2015, 5, 15088. [Google Scholar] [CrossRef] [Green Version]
- Ma, R.; Tao, Y.; Chen, Y.; Liu, T.; Luo, Z.; Guo, Y.; Xiao, Y.; Fang, J.; Zhang, G.; Li, X.; et al. Achieving 16.68% Efficiency Ternary As-Cast Organic Solar Cells. Sci. China Chem. 2021, 64, 581–589. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Marchuk, M.V.; Vorotnikova, N.A.; Vorotnikov, Y.A.; Kuratieva, N.V.; Stass, D.V.; Shestopalov, M.A. Optical Property Trends in a Family of {Mo6I8} Aquahydroxo Complexes. Dalton Trans. 2021, 50, 8794–8802. [Google Scholar] [CrossRef]
- Nur, A.S.M.; Sultana, M.; Mondal, A.; Islam, S.; Robel, F.N.; Islam, A.; Sumi, M.S.A. A Review on the Development of Elemental and Codoped TiO2 Photocatalysts for Enhanced Dye Degradation under UV–vis Irradiation. J. Water Process. Eng. 2022, 47, 102728. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic Activity Improvement and Application of UV-TiO2 Photocatalysis in Textile Wastewater Treatment: A Review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Xu, Q.L.; Zhang, L.Y.; Cheng, B.; Fan, J.J.; Yu, J.G. S-Scheme Heterojunction Photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Gharaei, S.K.; Abbasnejad, M.; Maezono, R. Bandgap Reduction of Photocatalytic TiO2 Nanotube by Cu Doping. Sci. Rep. 2018, 8, 14192. [Google Scholar] [CrossRef]
- Kashiwaya, S.; Morasch, J.; Streibel, V.; Toupance, T.; Jaegermann, W.; Klein, A. The Work Function of TiO2. Surfaces 2018, 1, 73–89. [Google Scholar] [CrossRef] [Green Version]
- Das, T.; Rocquefelte, X.; Jobic, S. Ab Initio Positioning of the Valence and Conduction Bands of Bulk Photocatalysts: Proposition of Absolute Reference Energy. J. Phys. Chem. C 2020, 124, 19426–19434. [Google Scholar] [CrossRef]
- Mansfeldova, V.; Zlamalova, M.; Tarabkova, H.; Janda, P.; Vorokhta, M.; Piliai, L.; Kavan, L. Work Function of TiO2 (Anatase, Rutile, and Brookite) Single Crystals: Effects of the Environment. J. Phys. Chem. C 2021, 125, 1902–1912. [Google Scholar] [CrossRef]










| Sample | Specific Surface Area, m2 g−1 | Vpore/cm3·g−1 | |
|---|---|---|---|
| BET | DFT | ||
| TiO2 | 253.2 | 277.0 | 0.406 |
| 10.1@TiO2 | 213.9 | 178.5 | 0.382 |
| 20.1@TiO2 | 213.2 | 186.6 | 0.349 |
| Sample | Rate Constant (keff), min−1 | |
|---|---|---|
| TiO2 | 0.02 | |
| n = 1 | n = 2 | |
| n0.1@TiO2 | 0.099 | 0.11 |
| n0.5@TiO2 | 0.10 | 0.075 |
| n1@TiO2 | 0.11 | 0.062 |
| n1.5@TiO2 | 0.096 | 0.025 |
| Ccat = 0.125 g L−1 | ||
| n = 1 | n = 2 | |
| n0.1@TiO2 | 0.055 | 0.026 |
| Ccat = 0.375 g L−1 | ||
| n = 1 | n = 2 | |
| n0.1@TiO2 | 0.047 | 0.081 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchuk, M.V.; Asanov, I.P.; Panafidin, M.A.; Vorotnikov, Y.A.; Shestopalov, M.A. Nano TiO2 and Molybdenum/Tungsten Iodide Octahedral Clusters: Synergism in UV/Visible-Light Driven Degradation of Organic Pollutants. Nanomaterials 2022, 12, 4282. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12234282
Marchuk MV, Asanov IP, Panafidin MA, Vorotnikov YA, Shestopalov MA. Nano TiO2 and Molybdenum/Tungsten Iodide Octahedral Clusters: Synergism in UV/Visible-Light Driven Degradation of Organic Pollutants. Nanomaterials. 2022; 12(23):4282. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12234282
Chicago/Turabian StyleMarchuk, Margarita V., Igor P. Asanov, Maxim A. Panafidin, Yuri A. Vorotnikov, and Michael A. Shestopalov. 2022. "Nano TiO2 and Molybdenum/Tungsten Iodide Octahedral Clusters: Synergism in UV/Visible-Light Driven Degradation of Organic Pollutants" Nanomaterials 12, no. 23: 4282. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12234282