Synthesis and Characterization of Highly Crystalline Bi-Functional Mn-Doped Zn2SiO4 Nanostructures by Low-Cost Sol–Gel Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Zn2SiO4 Nanoparticles
2.3. Measurement and Characterizations
3. Results and Discussion
3.1. Formation of α-Phase Zn2SiO4
3.2. Structural Analysis
3.3. Morphology Analysis
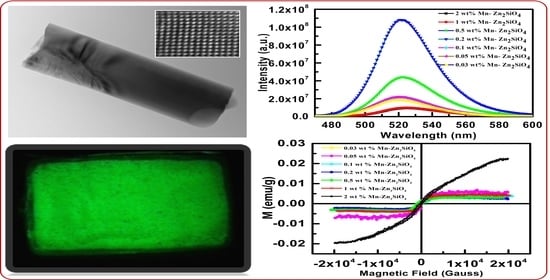
3.4. Photoluminescence Analysis
3.5. Magnetic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bharti, D.K.; Gupta, M.K.; Kumar, R.; Sathish, N.; Srivastava, A.K. Non-centrosymmetric zinc silicate-graphene based transparent flexible piezoelectric nanogenerator. Nano Energy 2020, 73, 104821. [Google Scholar] [CrossRef]
- Bharti, D.K.; Gupta, M.K.; Srivastava, A.K. Giant dielectric constant and band gap reduction in hydrothermal grown highly crystalline zinc silicate nanorods. Mater. Lett. 2018, 232, 66–69. [Google Scholar] [CrossRef]
- Bharti, D.K.; Veeralingam, S.; Badhulika, S. An ultra high performance, lead-free Bi2WO6:P(VDF-TrFE)-based triboelectric nanogenerator for self-powered sensors and smart electronic applications. Mater. Horiz. 2022, 9, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.; Jain, K.; Kumar, V.; Pant, R.P. Magnetic Fluid Based High Precision Temperature Sensor. IEEE Sens. J. 2017, 17, 2670–2675. [Google Scholar] [CrossRef]
- Pathak, S.; Zhang, R.; Gayen, B.; Kumar, V.; Zhang, H.; Pant, R.P.; Wang, X. Ultra-low friction self-levitating nanomagnetic fluid bearing for highly efficient wind energy harvesting. Sustain. Energy Technol. Assess. 2022, 52, 102024. [Google Scholar] [CrossRef]
- Verma, R.; Pathak, S.; Srivastava, A.K.; Prawer, S.; Tomljenovic-Hanic, S. ZnO nanomaterials: Green synthesis, toxicity evaluation and new insights in biomedical applications. J. Alloys Compd. 2021, 876, 160175. [Google Scholar] [CrossRef]
- Verma, R.; Gangwar, J.; Srivastava, A.K. Multiphase TiO2 nanostructures: A review of efficient synthesis, growth mechanism, probing capabilities, and applications in bio-safety and health. RSC Adv. 2017, 7, 44199–44224. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Rani, S.; Kumar, A.; Tawale, J.; Srivastava, R.; Singh, B.P.; Pathak, S.; Wang, X.; Singh, V.N. Broadband (NIR-Vis-UV) photoresponse of annealed SnSe films and effective oxidation passivation using Si protective layer. Mater. Res. Bull. 2022, 153, 111913. [Google Scholar] [CrossRef]
- Gbadamasi, S.; Mohiuddin, M.; Krishnamurthi, V.; Verma, R.; Khan, M.W.; Pathak, S.; Kalantar-Zadeh, K.; Mahmood, N. Interface chemistry of two-dimensional heterostructures—Fundamentals to applications. Chem. Soc. Rev. 2021, 50, 4684–4729. [Google Scholar] [CrossRef]
- Verma, R.; Pathak, S.; Dey, K.K.; Sikarwar, S.; Yadav, B.C.; Srivastava, A.K. Facile synthesized zinc oxide nanorod film humidity sensor based on variation in optical transmissivity. Nanoscale Adv. 2022, 4, 2902–2912. [Google Scholar] [CrossRef]
- Goswami, L.; Aggarwal, N.; Verma, R.; Bishnoi, S.; Husale, S.; Pandey, R.; Gupta, G. Graphene quantum dots sensitized ZnO-Nanorods/GaN-Nanotowers heterostructure based high performance UV Photodetector. ACS Appl. Mater. Interfaces 2020, 12, 47038–47047. [Google Scholar] [CrossRef] [PubMed]
- Goswami, L.; Aggarwal, N.; Singh, M.; Verma, R.; Vashishtha, P.; Jain, S.K.; Tawale, J.; Pandey, R.; Gupta, G. GaN Nanotowers Grown on Si (111) and Functionalized with Au Nanoparticles and ZnO Nanorods for Highly Responsive UV Photodetectors. ACS Appl. Nano Mater. 2020, 3, 8104–8116. [Google Scholar] [CrossRef]
- Singh, D.; Kundu, V.S.; Dhiman, R.L.; Gangwar, J. Structural and morphological study of zinc doped tin oxide nanoparticles synthesized via sol-gel technique. AIP Conf. Proc. 2018, 2006, 030017. [Google Scholar]
- Kumar, P.; Pathak, S.; Singh, A.; Jain, K.; Khanduri, H.; Wang, L.; Kim, S.-K.; Pant, R.P. Observation of intrinsic fluorescence in cobalt ferrite magnetic nanoparticles by Mn2+ substitution and tuning the spin dynamics by cation distribution. J. Mater. Chem. C 2022, 10, 12652–12679. [Google Scholar] [CrossRef]
- Pathak, S.; Zhang, R.; Bun, K.; Zhang, H.; Gayen, B.; Wang, X. Development of a novel wind to electrical energy converter of passive ferrofluid levitation through its parameter modelling and optimization. Sustain. Energy Technol. Assess. 2021, 48, 101641. [Google Scholar] [CrossRef]
- Bakshi, P.; Pappu, A.; Kumar Bharti, D. Transformation of flue gas desulfurization (FGD) gypsum to β-CaSO4·0.5H2O whiskers using facile water treatment. Mater. Lett. 2021, 308, 131177. [Google Scholar] [CrossRef]
- Dai, P.; Xu, Z.; Yu, X.; Wang, Y.; Zhang, L.; Li, G.; Sun, Z.; Liu, X.; Wu, M. Mesoporous hollow Zn2SiO4:Mn2+ nanospheres: The study of photoluminescence and adsorption properties. Mater. Res. Bull. 2015, 61, 76–82. [Google Scholar] [CrossRef]
- Basavaraj, R.B.; Nagabhushana, H.; Daruka Prasad, B.; Sharma, S.C.; Prashantha, S.C.; Nagabhushana, B.M. A single host white light emitting Zn2SiO4:Re3+ (Eu, Dy, Sm) phosphor for LED applications. Opt.—Int. J. Light Electron Opt. 2015, 126, 1745–1756. [Google Scholar] [CrossRef]
- Qu, J.; Cao, C.-Y.; Hong, Y.-L.; Chen, C.-Q.; Zhu, P.-P.; Song, W.-G.; Wu, Z.-Y. New hierarchical zinc silicate nanostructures and their application in lead ion adsorption. J. Mater. Chem. 2012, 22, 3562–3567. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, M.; Li, Y.; Sun, F.; Yang, J.; Wang, S. Synthesis and electrochemical properties of Zn2SiO4 nano/mesorods. Mater. Lett. 2013, 100, 89–92. [Google Scholar] [CrossRef]
- El Ghoul, J.; Omri, K.; El Mir, L.; Barthou, C.; Alaya, S. Sol-gel synthesis and luminescent properties of SiO2/Zn2SiO4 and SiO2/Zn2SiO4:V composite materials. J. Lumin. 2012, 132, 2288–2292. [Google Scholar] [CrossRef]
- Su, K.; Tilley, T.D.; Sailor, M.J. Molecular and Polymer Precursor Routes to Manganese-Doped Zinc Orthosilicate Phosphors. J. Am. Chem. Soc. 1996, 118, 3459–3468. [Google Scholar] [CrossRef]
- Li, Q.H.; Komarneni, S.; Roy, R. Control of morphology of Zn2SiO4 by hydrothermal preparation. J. Mater. Sci. 1995, 30, 2358–2363. [Google Scholar] [CrossRef]
- Kang, Y.C.; Park, S.B. Zn2SiO4:Mn phosphor particles prepared by spray pyrolysis using a filter expansion aerosol generator. Mater. Res. Bull. 2000, 35, 1143–1151. [Google Scholar] [CrossRef]
- Krsmanović Whiffen, R.; Antić, Ž.; Marinović-Cincović, M.; Dramicanin, M. Samarium and terbium doped Zn2SiO4 phosphors obtained by polymer supported sol-gel synthesis. J. Optoelectron. Adv. Mater. 2009, 1, 37–41. [Google Scholar]
- Wang, H.; Ma, Y.; Yi, G.; Chen, D. Synthesis of Mn-doped Zn2SiO4 rodlike nanoparticles through hydrothermal method. Mater. Chem. Phys. 2003, 82, 414–418. [Google Scholar] [CrossRef]
- Pozas, R.; Orera, V.M.; Ocaña, M. Hydrothermal synthesis of Co-doped willemite powders with controlled particle size and shape. J. Eur. Ceram. Soc. 2005, 25, 3165–3172. [Google Scholar] [CrossRef]
- Omri, K.; Lassaad, E.M.; Dahman, H.; Barthou, C. Synthesis and Luminescence Properties of Yellow-emitting SiO2/Zn2SiO4:Mn Nanocomposite. Sens. Tranducers 2014, 27, 295–298. [Google Scholar]
- Gao, G.; Reibstein, S.; Peng, M.; Wondraczek, L. Tunable dual-mode photoluminescence from nanocrystalline Eu-doped Li2ZnSiO4 glass ceramic phosphors. J. Mater. Chem. 2011, 21, 3156–3161. [Google Scholar] [CrossRef]
- Omri, K.; Alyamani, A.; Mir, L.E. Photoluminescence and cathodoluminescence of Mn doped zinc silicate nanophosphors for green and yellow field emissions displays. Appl. Phys. A 2018, 124, 215–221. [Google Scholar] [CrossRef]
- Hafeez, M.; Ali, A.; Manzoor, S.; Bhatti, A.S. Anomalous optical and magnetic behavior of multi-phase Mn doped Zn2SiO4 nanowires: A new class of dilute magnetic semiconductors. Nanoscale 2014, 6, 14845–14855. [Google Scholar] [CrossRef]
- Wan, J.; Wang, Z.; Chen, X.; Mu, L.; Yu, W.; Qian, Y. Controlled synthesis and relationship between luminescent properties and shape/crystal structure of Zn2SiO4:Mn2+ phosphor. J. Lumin. 2006, 121, 32–38. [Google Scholar] [CrossRef]
- El Ghoul, J.; Barthou, C.; Saadoun, M.; El Mir, L. Synthesis and optical characterization of SiO2/Zn2SiO4:Mn nanocomposite. Phys. B Condens. Matter 2010, 405, 597–601. [Google Scholar] [CrossRef]
- Kumar, P.; Khanduri, H.; Pathak, S.; Singh, A.; Basheed, G.A.; Pant, R.P. Temperature selectivity for single phase hydrothermal synthesis of PEG-400 coated magnetite nanoparticles. Dalton Trans. 2020, 49, 8672–8683. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Pathak, S.; Singh, A.; Khanduri, H.; Basheed, G.A.; Wang, L.; Pant, R.P. Microwave spin resonance investigation on the effect of the post-processing annealing of CoFe2O4 nanoparticles. Nanoscale Adv. 2020, 2, 1939–1948. [Google Scholar] [CrossRef] [Green Version]
- Pathak, S.; Verma, R.; Kumar, P.; Singh, A.; Singhal, S.; Sharma, P.; Jain, K.; Pant, R.P.; Wang, X. Facile Synthesis, Static, and Dynamic Magnetic Characteristics of Varying Size Double-Surfactant-Coated Mesoscopic Magnetic Nanoparticles Dispersed Stable Aqueous Magnetic Fluids. Nanomaterials 2021, 11, 3009. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.; Verma, R.; Singhal, S.; Chaturvedi, R.; Kumar, P.; Sharma, P.; Pant, R.P.; Wang, X. Spin dynamics investigations of multifunctional ambient scalable Fe3O4 surface decorated ZnO magnetic nanocomposite using FMR. Sci. Rep. 2021, 11, 3799. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Basheed, G.A.; Jain, K.; Pathak, S.; Pant, R.P. Dipolar Interaction and Magneto-Viscoelasticity in Nanomagnetic Fluid. J. Nanosci. Nanotechnol. 2018, 18, 2746–2751. [Google Scholar]
- Jain, K.; Pathak, S.; Pant, R.P. Enhanced magnetic properties in ordered oriented ferrofibres. RSC Adv. 2016, 6, 70943–70946. [Google Scholar] [CrossRef]
- Tripathi, N.; Akai, T. Structural designing of Zn2SiO4:Mn nanocrystals by co-doping of alkali metal ions in mesoporous silica channels for enhanced emission efficiency with short decay time. RSC Adv. 2021, 11, 36348–36353. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.H.; Han, K.S.; Lee, B.H. Synthesis and Formation Mechanism of Mn-Doped Zn2SiO4 Brown Pigment. Mater. Sci. Forum 2011, 695, 295–298. [Google Scholar] [CrossRef]
- Kwon, M.S.; Kim, C.J.; Park, H.L.; Kim, T.W.; Lee, H.S. Sol–gel synthesis and green luminescence of nanocrystalline Zn2SiO4:Mn phosphor. J. Mater. Sci. 2005, 40, 4089–4091. [Google Scholar] [CrossRef]
- Hu, T.; Lin, H.; Xu, J.; Wang, B.; Wang, J.; Wang, Y. Color-tunable persistent luminescence in oxyfluoride glass and glass ceramic containing Mn2+:α-Zn2SiO4 nanocrystals. J. Mater. Chem. C 2017, 5, 1479–1487. [Google Scholar] [CrossRef]
- Yoon, K.H.; Kim, J.H. Optical Properties and Photoluminescent Characteristics of Manganese-doped Zinc Silicate Thin Films. J. Korean Phys. Soc. 2011, 6, 1668–1671. [Google Scholar] [CrossRef]
- Lu, Q.; Wang, P.; Li, J. Structure and luminescence properties of Mn-doped Zn2SiO4 prepared with extracted mesoporous silica. Mater. Res. Bull. 2011, 46, 791–795. [Google Scholar] [CrossRef]
- Omri, K.; Lemine, O.M.; El Mir, L. Mn doped zinc silicate nanophosphor with bifunctionality of green-yellow emission and magnetic properties. Ceram. Int. 2017, 43, 6585–6591. [Google Scholar] [CrossRef]
- Zheng, W.L.; Yang, W. Hydrothermal Synthesis of Diluted Magnetic Zn1-xMnxO Semiconductor. Appl. Mech. Mater. 2013, 313–314, 184–187. [Google Scholar] [CrossRef]
- Jain, K.; Pathak, S.; Kumar, P.; Singh, A.; Pant, R.P. Dynamic magneto-optical inversion in magnetic fluid using NanoMOKE. J. Magn. Magn. Mater. 2019, 475, 782–786. [Google Scholar] [CrossRef]
- Kumar, P.; Pathak, S.; Jain, K.; Singh, A.; Basheed, G.A.; Pant, R.P. Low-temperature large-scale hydrothermal synthesis of optically active PEG-200 capped single domain MnFe2O4 nanoparticles. J. Alloys Compd. 2022, 904, 163992. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bharti, D.K.; Verma, R.; Rani, S.; Agarwal, D.; Mehra, S.; Gangwar, A.K.; Gupta, B.K.; Singh, N.; Srivastava, A.K. Synthesis and Characterization of Highly Crystalline Bi-Functional Mn-Doped Zn2SiO4 Nanostructures by Low-Cost Sol–Gel Process. Nanomaterials 2023, 13, 538. https://0-doi-org.brum.beds.ac.uk/10.3390/nano13030538
Bharti DK, Verma R, Rani S, Agarwal D, Mehra S, Gangwar AK, Gupta BK, Singh N, Srivastava AK. Synthesis and Characterization of Highly Crystalline Bi-Functional Mn-Doped Zn2SiO4 Nanostructures by Low-Cost Sol–Gel Process. Nanomaterials. 2023; 13(3):538. https://0-doi-org.brum.beds.ac.uk/10.3390/nano13030538
Chicago/Turabian StyleBharti, Dhiraj Kumar, Rajni Verma, Sonam Rani, Daksh Agarwal, Sonali Mehra, Amit Kumar Gangwar, Bipin Kumar Gupta, Nidhi Singh, and Avanish Kumar Srivastava. 2023. "Synthesis and Characterization of Highly Crystalline Bi-Functional Mn-Doped Zn2SiO4 Nanostructures by Low-Cost Sol–Gel Process" Nanomaterials 13, no. 3: 538. https://0-doi-org.brum.beds.ac.uk/10.3390/nano13030538