Mesoporous Cobalt Oxide (CoOx) Nanowires with Different Aspect Ratios for High Performance Hybrid Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Self-Supported CoOx
2.2. Material Characterizations
2.3. Electrochemical Measurements
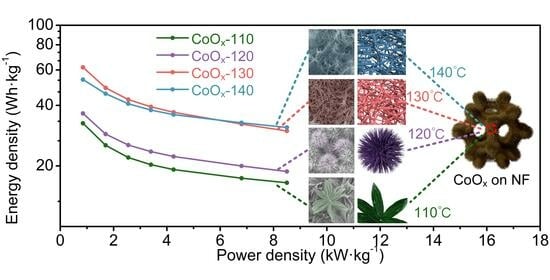
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zeng, Q.; Lai, Y.; Jiang, L.; Liu, F.; Hao, X.; Wang, L.; Green, M.A. Integrated Photorechargeable Energy Storage System: Next-Generation Power Source Driving the Future. Adv. Energy Mater. 2020, 10, 1903930. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy Storage: The Future Enabled by Nanomaterials. Science 2019, 366, eaan8285. [Google Scholar] [CrossRef]
- Xia, X.; Pan, J.H.; Pan, X.; Hu, L.; Yao, J.; Ding, Y.; Wang, D.; Ye, J.; Dai, S. Photochemical Conversion and Storage of Solar Energy. ACS Energy Lett. 2019, 4, 405–410. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Pazhamalai, P.; Manoharan, S.; Liyakath Ali, N.U.H.; Kim, S.J. Recent Trends, Challenges, and Perspectives in Piezoelectric-Driven Self-Chargeable Electrochemical Supercapacitors. Carbon Energy 2022, 4, 833–855. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Photo-Powered Integrated Supercapacitors: A Review on Recent Developments, Challenges and Future Perspectives. J. Mater. Chem. A 2021, 9, 8248–8278. [Google Scholar]
- Peng, H.; Gao, X.; Sun, K.; Xie, X.; Ma, G.; Zhou, X.; Lei, Z. Physically Cross-Linked Dual-Network Hydrogel Electrolyte with High Self-Healing Behavior and Mechanical Strength for Wide-Temperature Tolerant Flexible Supercapacitor. Chem. Eng. J. 2021, 422, 130353. [Google Scholar] [CrossRef]
- Qin, K.; Baucom, J.; Diao, L.; Lu, Y.; Zhao, N. Compacted N-Doped 3D Bicontinuous Nanoporous Graphene/Carbon Nanotubes@Ni-Doped MnO2 Electrode for Ultrahigh Volumetric Performance All-Solid-State Supercapacitors at Wide Temperature Range. Small 2022, 18, 2203166. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, S.; Mitchell, J.B.; Wang, R.; Zhan, C.; Jiang, D.E.; Presser, V.; Augustyn, V. Pseudocapacitance: From Fundamental Understanding to High Power Energy Storage Materials. Chem. Rev. 2020, 120, 6738–6782. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Li, N.; Jin, S.; Yang, G.; Wang, C. All-Solid-State Symmetric Supercapacitor Based on Co3O4 Nanoparticles on Vertically Aligned Graphene. ACS Nano 2015, 9, 5310–5317. [Google Scholar] [CrossRef]
- Zhou, H.; Kang, M.; Xie, B.; Wen, P.; Zhao, N. L-Alanine Mediated Controllable Synthesis: Ultrathin Co3O4 Nanosheets@Ni Foam for High Performance Supercapacitors. J. Alloys Compd. 2021, 874, 160030. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, W.; Wang, S.; Du, W.; Zhang, H.; Ding, C.; Du, Y.; Zhu, L. Facile Synthesis of Homogeneous Core-Shell Co3O4 Mesoporous Nanospheres as High Performance Electrode Materials for Supercapacitor. J. Alloys Compd. 2019, 774, 137–144. [Google Scholar] [CrossRef]
- Paliwal, M.K.; Meher, S.K. Sedgelike Porous Co3O4 Nanoarrays as a Novel Positive Electrode Material for Co3O4||Bi2O3 Asymmetric Supercapacitors. ACS Appl. Nano Mater. 2019, 2, 5573–5586. [Google Scholar] [CrossRef]
- Wang, Q.; Zhong, T.; Wang, Z. Plasma-Engineered N-CoOx Nanowire Array as a Bifunctional Electrode for Supercapacitor and Electrocatalysis. Nanomaterials 2022, 12, 2984. [Google Scholar] [CrossRef] [PubMed]
- Albaqami, M.D.; Medany, S.S.; Nafady, A.; Ibupoto, M.H.; Willander, M.; Tahira, A.; Aftab, U.; Vigolo, B.; Ibupoto, Z.H. The Fast Nucleation/Growth of Co3O4 Nanowires on Cotton Silk: The Facile Development of a Potentiometric Uric Acid Biosensor. RSC Adv. 2022, 12, 18321–18332. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Yan, L.; Huang, H.; Yan, F.; Liang, X.; Xu, S.; Lan, Z.; Zhou, W.; Guo, J. The Hetero-Structured Nanoarray Construction of Co3O4 Nanowires Anchored on Nanoflakes as a High-Performance Electrode for Supercapacitors. Appl. Surf. Sci. 2021, 538, 147932. [Google Scholar] [CrossRef]
- Ali, A.; Ammar, M.; Hameed, I.; Ali, M.; Tayyab, M.; Mujahid, R.; Ali, I.; Zia-ul-Haq, M.; Ashraf, M. Hydrothermally Synthesized Cobalt Oxide Nanowires on Nickel Foam for High-Performance Energy-Storage Applications. J. Electrochem. Soc. 2020, 167, 100509. [Google Scholar] [CrossRef]
- Guo, C.; Yin, M.; Wu, C.; Li, J.; Sun, C.; Jia, C.; Li, T.; Hou, L.; Wei, Y. Highly Stable Gully-Network Co3O4 Nanowire Arrays as Battery-Type Electrode for Outstanding Supercapacitor Performance. Front. Chem. 2018, 6, 636. [Google Scholar] [CrossRef] [PubMed]
- Rakhi, R.B.; Chen, W.; Cha, D.; Alshareef, H.N. Substrate Dependent Self-Organization of Mesoporous Cobalt Oxide Nanowires with Remarkable Pseudocapacitance. Nano Lett. 2012, 12, 2559–2567. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, T.W.; Chen, L.; Chen, Y.F.; Yang, B.R.; Luo, Y.H.; Liu, G.S. Self-Assembly, Alignment, and Patterning of Metal Nanowires. Nanoscale Horiz. 2022, 7, 1299–1339. [Google Scholar] [PubMed]
- Shu, T.; Wang, H.; Li, Q.; Feng, Z.; Wei, F.; Yao, K.X.; Sun, Z.; Qi, J.; Sui, Y. Highly Stable Co3O4 Nanoparticles/Carbon Nanosheets Array Derived from Flake-Like ZIF-67 as an Advanced Electrode for Supercapacacitor. Chem. Eng. J. 2021, 419, 129631. [Google Scholar] [CrossRef]
- Yun, M.; Ma, Y.; Cai, Z.; Ji, H.; Han, J.; Wang, M.; Tong, Z.; Xiao, L.; Jia, S.; Chen, X. Holey Graphene Interpenetrating Networks for Boosting High-Capacitive Co3O4 Electrodes Via an Electrophoretic Deposition Process. Ceram. Int. 2021, 47, 27210–27216. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wang, N.; Tang, X.Z.; Pillai, S.C.; Hu, W.C. High Performance Supercapacitors Using Selenium Partially Reduced Co3O4 on Carbon Cloth Electrode with 3D Interconnected Architecture Nanowires. Appl. Surf. Sci. 2022, 605, 154785. [Google Scholar] [CrossRef]
- Li, J.; Jiang, W.; Wang, D. Synthesis of Co3O4@CNTs with Oxygen Vacancies on Nickel Foam for Improved Performance of Asymmetric Supercapacitor Electrode. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130750. [Google Scholar] [CrossRef]
- Panahi, M.; Ghorbani, M.; Lashkenari, M.S. Construction of CO3O4 Derived ZIF/GO Electrode for Outstanding Stability in Supercapacitors Devices. Int. J. Hydrog. Energy 2022, 47, 9800–9809. [Google Scholar] [CrossRef]
- Deng, J.; Kang, L.; Bai, G.; Li, Y.; Li, P.; Liu, X.; Yang, Y.; Gao, F.; Liang, W. Solution Combustion Synthesis of Cobalt Oxides (Co3O4 and Co3O4/CoO) Nanoparticles as Supercapacitor Electrode Materials. Electrochim. Acta 2014, 132, 127–135. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Chang, Z.; Yang, Y.; Wu, Y.; Liu, X. Co3O4@MWCNT Nanocable as Cathode with Superior Electrochemical Performance for Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 2280–2285. [Google Scholar] [CrossRef]
- Wang, H.; Qing, C.; Guo, J.; Aref, A.A.; Sun, D.; Wang, B.; Tang, Y. Highly Conductive Carbon–CoO Hybrid Nanostructure Arrays with Enhanced Electrochemical Performance for Asymmetric Supercapacitors. J. Mater. Chem. A 2014, 2, 11776–11783. [Google Scholar] [CrossRef]
- Cheng, M.; Duan, S.; Fan, H.; Su, X.; Cui, Y.; Wang, R. Core@Shell CoO@Co3O4 nanocrystals Assembling Mesoporous Microspheres for High Performance Asymmetric Supercapacitors. Chem. Eng. J. 2017, 327, 100–108. [Google Scholar] [CrossRef]
- Pang, M.; Long, G.; Jiang, S.; Ji, Y.; Han, W.; Wang, B.; Liu, X.; Xi, Y.; Wang, D.; Xu, F. Ethanol-Assisted Solvothermal Synthesis of Porous Nanostructured Cobalt Oxides (CoO/Co3O4) for High-Performance Supercapacitors. Chem. Eng. J. 2015, 280, 377–384. [Google Scholar] [CrossRef]
- Dhakal, G.; Mohapatra, D.; Tamang, T.L.; Lee, M.; Lee, Y.R.; Shim, J.-J. Redox-Additive Electrolyte–Driven Enhancement of the Electrochemical Energy Storage Performance of Asymmetric Co3O4//Carbon Nano-Onions Supercapacitors. Energy 2021, 218, 119436. [Google Scholar] [CrossRef]
- Zhang, Z.; Hao, J.; Yang, W.; Lu, B.; Ke, X.; Zhang, B.; Tang, J. Porous Co3O4 Nanorods-Reduced Graphene Oxide with Intrinsic Peroxidase-Like Activity and Catalysis in the Degradation of Methylene Blue. ACS Appl. Mater. Interfaces 2013, 5, 3809–3815. [Google Scholar] [CrossRef]
- Guo, W.; Luo, H.; Fang, D.; Jiang, Z.; Chi, J.; Shangguan, W. In Situ Revealing the Reconstruction Behavior of Monolayer Rocksalt CoO Nanosheet as Water Oxidation Catalyst. J. Energy Chem. 2022, 70, 373–381. [Google Scholar] [CrossRef]
- Hu, Q.; Gu, Z.; Zheng, X.; Zhang, X. Three-Dimensional Co3O4@NiO Hierarchical Nanowire Arrays for Solid-State Symmetric Supercapacitor with Enhanced Electrochemical Performances. Chem. Eng. J. 2016, 304, 223–231. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Z.; Zhao, B.; Cheng, Y.; Zhang, L.; Wu, H.-H.; Wang, M.-S.; Dai, S.; Zhang, K.; Ding, D.; et al. Design and Understanding of Dendritic Mixed-Metal Hydroxide Nanosheets@N-Doped Carbon Nanotube Array Electrode for High-Performance Asymmetric Supercapacitors. Energy Stor. Mater. 2019, 16, 632–645. [Google Scholar] [CrossRef]
- Xiong, S.; Yuan, C.; Zhang, X.; Xi, B.; Qian, Y. Controllable Synthesis of Mesoporous Co3O4 Nanostructures with Tunable Morphology for Application in Supercapacitors. Chemistry 2009, 15, 5320–5326. [Google Scholar] [CrossRef]
- Qu, C.; Zhao, B.; Jiao, Y.; Chen, D.; Dai, S.; Deglee, B.M.; Chen, Y.; Walton, K.S.; Zou, R.; Liu, M. Functionalized Bimetallic Hydroxides Derived From Metal–Organic Frameworks For High-Performance Hybrid Supercapacitor With Exceptional Cycling Stability. ACS Energy Lett. 2017, 2, 1263–1269. [Google Scholar] [CrossRef]
- Mukhiya, T.; Tiwari, A.P.; Chhetri, K.; Kim, T.; Dahal, B.; Muthurasu, A.; Kim, H.Y. A Metal–Organic Framework Derived Cobalt Oxide/Nitrogen-Doped Carbon Nanotube Nanotentacles On Electrospun Carbon Nanofiber for Electrochemical Energy Storage. Chem. Eng. J. 2021, 420, 129679. [Google Scholar] [CrossRef]
- Song, W.-W.; Wang, B.; Li, C.-N.; Wang, S.-M.; Han, Z.-B. 3D Hierarchical Core–Shell Spiny Globe Shaped Co2P@Ni2P/NiCo2O4@CoO for Asymmetric Supercapacitors. J. Mater. Chem. A 2022, 10, 3710–3721. [Google Scholar] [CrossRef]
- Chen, M.; Lu, Y.; Li, W.; Qi, P.; Liu, G.; Wang, S.; Chen, Z.; Tang, Y. In-situ Transformation Constructs CoTe/Co/CoO Nanosheet Arrays with Rich Grain Boundaries to Enhance Electrochemical Performance. Electrochim. Acta 2022, 413, 140101. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Xu, C.; Jiang, H.; Li, C.; Zhang, L.; Lin, J.; Shen, Z.X. Advanced Energy Storage Devices: Basic Principles, Analytical Methods, and Rational Materials Design. Adv. Sci. 2018, 5, 1700322. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive Oxide Materials for High-Rate Electrochemical Energy Storage. Energy Environ. Sci. 2014, 4, 1597–1614. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, J.; Wang, N.; Cheng, H.; Tang, X.; Komarneni, S.; Hu, W. Significantly Improved Conductivity of Spinel Co3O4 Porous Nanowires Partially Substituted by Sn in Tetrahedral Sites for High-Performance Quasi-Solid-State Supercapacitors. J. Mater. Chem. A 2021, 9, 7005–7017. [Google Scholar] [CrossRef]
- Chen, H.; Du, X.; Sun, J.; Wu, R.; Wang, Y.; Xu, C. Template-Free Synthesis of Novel Co3O4 Micro-Bundles Assembled with Flakes for High-Performance Hybrid Supercapacitors. Ceram. Int. 2021, 47, 716–724. [Google Scholar] [CrossRef]
- Guo, W.; Lian, X.; Tian, Y.; Yang, T.; Wang, S. Facile Fabrication 1D/2D/3D Co3O4 Nanostructure in Hydrothermal Synthesis for Enhanced Supercapacitor Performance. J. Energy Storage 2021, 38, 102586. [Google Scholar] [CrossRef]
- Chen, H.; Xue, C.; Hai, Z.; Cui, D.; Liu, M.; Li, Y.; Zhang, W. Facile Synthesis of 3D Gem Shape Co3O4 with Mesoporous Structure as Electrode for High-Performance Supercapacitors. J. Alloys Compd. 2020, 819, 152939. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Chen, C.; An, C.; Xu, Y.; Dong, Y.; Zhang, Q.; Wang, Y.; Jiao, L.; Yuan, H. Facile Synthesis of Diverse Transition Metal Oxide Nanoparticles and Electrochemical Properties. Inorg. Chem. Front. 2016, 3, 1048–1057. [Google Scholar] [CrossRef]
- Shen, B.; Liao, X.; Zhang, X.; Ren, H.-T.; Lin, J.-H.; Lou, C.-W.; Li, T.-T. Synthesis of Nb2C MXene-Based 2D Layered Structure Electrode Material for High-Performance Battery-Type Supercapacitors. Electrochim. Acta 2022, 413, 140144. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Kumar, Y.A.; Mani, G.; Nallapureddy, R.R.; Parvathala, A.; Albaqami, M.D.; Karami, A.M.; Joo, S.W. A Novel Hybridized Needle-Like Co3O4/N-CNO Composite for Superior Energy Storage Asymmetric Supercapacitors. J. Alloys Compd. 2022, 908, 164447. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, H.; Ma, Y.; Cai, Z.; Yun, M.; Han, J.; Tong, Z.; Wang, M.; Suhr, J.; Xiao, L.; Jia, S.; et al. Mesoporous Cobalt Oxide (CoOx) Nanowires with Different Aspect Ratios for High Performance Hybrid Supercapacitors. Nanomaterials 2023, 13, 749. https://0-doi-org.brum.beds.ac.uk/10.3390/nano13040749
Ji H, Ma Y, Cai Z, Yun M, Han J, Tong Z, Wang M, Suhr J, Xiao L, Jia S, et al. Mesoporous Cobalt Oxide (CoOx) Nanowires with Different Aspect Ratios for High Performance Hybrid Supercapacitors. Nanomaterials. 2023; 13(4):749. https://0-doi-org.brum.beds.ac.uk/10.3390/nano13040749
Chicago/Turabian StyleJi, Haomin, Yifei Ma, Zhuo Cai, Micun Yun, Jiemin Han, Zhaomin Tong, Mei Wang, Jonghwan Suhr, Liantuan Xiao, Suotang Jia, and et al. 2023. "Mesoporous Cobalt Oxide (CoOx) Nanowires with Different Aspect Ratios for High Performance Hybrid Supercapacitors" Nanomaterials 13, no. 4: 749. https://0-doi-org.brum.beds.ac.uk/10.3390/nano13040749