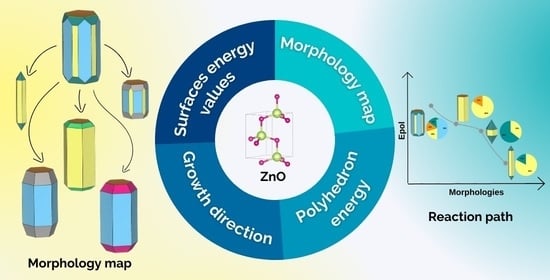
Back to the Basics: Probing the Role of Surfaces in the Experimentally Observed Morphological Evolution of ZnO
Abstract
:1. Introduction
2. Theoretical Methods
3. Results and Discussion
3.1. A computational Road to Morphology
3.2. Where Will This Road Take Us?
3.3. Which Way Does the Morphology Go?
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gouveia, A.F.; Gracia, L.; Longo, E.; San-Miguel, M.A.; Andres, J. Modulating the properties of multifunctional semiconductors by means of morphology: Theory meets experiments. Comput. Mater. Sci. 2021, 188, 110217. [Google Scholar] [CrossRef]
- Assis, M.; de Foggi, C.C.; Teodoro, V.; da Costa, J.P.D.; Silva, C.E.; Robeldo, T.; Caperucci, P.F.; Vergani, C.E.; Borra, R.C.; Sorribes, I.; et al. Surface-dependent photocatalytic and biological activities of Ag2CrO4: Integration of experiment and simulation. Appl. Surf. Sci. 2021, 545, 148964. [Google Scholar] [CrossRef]
- Jiang, W.; Xia, Y.; Pan, A.; Luo, Y.; Su, Y.; Zhao, S.; Wang, T.; Zhao, L. Facet-Dependent Gas Adsorption Selectivity on ZnO: A DFT Study. Chemosensors 2022, 10, 436. [Google Scholar] [CrossRef]
- Mora-Fonz, D.; Buckeridge, J.; Logsdail, A.J.; Scanlon, D.O.; Sokol, A.A.; Woodley, S.; Catlow, C.R.A. Morphological Features and Band Bending at Nonpolar Surfaces of ZnO. J. Phys. Chem. C 2015, 119, 11598–11611. [Google Scholar] [CrossRef]
- Mora-Fonz, D.; Lazauskas, T.; Farrow, M.R.; Catlow, C.R.A.; Woodley, S.M.; Sokol, A.A. Why Are Polar Surfaces of ZnO Stable? Chem. Mater. 2017, 29, 5306–5320. [Google Scholar] [CrossRef] [Green Version]
- Spencer, M.J.S. Gas sensing applications of 1D-nanostructured zinc oxide: Insights from density functional theory calculations. Prog. Mater. Sci. 2012, 57, 437–486. [Google Scholar] [CrossRef]
- Byzynski, G.; Melo, C.; Volanti, D.P.; Ferrer, M.M.; Gouveia, A.F.; Ribeiro, C.; Andres, J.; Longo, E. The interplay between morphology and photocatalytic activity in ZnO and N-doped ZnO crystals. Mater. Des. 2017, 120, 363–375. [Google Scholar] [CrossRef] [Green Version]
- Araujo, E.A.; Nobre, F.X.; Sousa, G.D.; Cavalcante, L.S.; Santos, M.; Souza, F.L.; de Matos, J.M.E. Synthesis, growth mechanism, optical properties and catalytic activity of ZnO microcrystals obtained via hydrothermal processing. RSC Adv. 2017, 7, 24263–24281. [Google Scholar] [CrossRef] [Green Version]
- Debroye, E.; Van Loon, J.; Yuan, H.F.; Janssen, K.P.F.; Lou, Z.Z.; Kim, S.; Majima, T.; Roeffaers, M.B.J. Facet-Dependent Photoreduction on Single ZnO Crystals. J. Phys. Chem. Lett. 2017, 8, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Samadi, M.; Zirak, M.; Naseri, A.; Kheirabadi, M.; Ebrahimi, M.; Moshfegh, A.Z. Design and tailoring of one-dimensional ZnO nanomaterials for photocatalytic degradation of organic dyes: A review. Res. Chem. Intermed. 2019, 45, 2197–2254. [Google Scholar] [CrossRef]
- Shakil, M.R.; Meguerdichian, A.G.; Tasnim, H.; Shirazi-Amin, A.; Seraji, M.S.; Suib, S.L. Syntheses of ZnO with Different Morphologies: Catalytic Activity toward Coumarin Synthesis via the Knoevenagel Condensation Reaction. Inorg. Chem. 2019, 58, 5703–5714. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Cai, L.; Wu, Y.; Ji, M.; Jiang, H.; Chen, J. Dissection of the antibacterial mechanism of zinc oxide nanoparticles with manipulable nanoscale morphologies. J. Hazard. Mater. 2022, 430, 128436. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Ma, J.; Peng, Y.; Wang, A. A review on bidirectional analogies between the photocatalysis and antibacterial properties of ZnO. J. Alloy. Compd. 2019, 783, 898–918. [Google Scholar] [CrossRef]
- He, X.; Yang, Y.; Li, Y.; Chen, J.; Yang, S.; Liu, R.; Xu, Z. Effects of structure and surface properties on the performance of ZnO towards photocatalytic degradation of methylene blue. Appl. Surf. Sci. 2022, 599, 153898. [Google Scholar] [CrossRef]
- Mohapatra, B.; Choudhary, S.; Mohapatra, S.; Sharma, N. Facile preparation and antibacterial activity of zinc oxide nanobullets. Mater. Today Commun. 2023, 34, 105083. [Google Scholar] [CrossRef]
- Gao, P.X.; Wang, Z.L. Mesoporous polyhedral cages and shells formed by textured self-assembly of ZnO nanocrystals. J. Am. Chem. Soc. 2003, 125, 11299–11305. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.P.; Zheng, Z.F.; Zhu, H.Y.; Pan, G.L.; Bao, J.L.; Wu, F.; Song, D.Y. Rotor-like ZnO by epitaxial growth under hydrothermal conditions. Chem. Commun. 2004, 1428–1429. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.H.; Lin, W.J.; Wu, M.M.; Xu, N.S.; Yang, X.F.; Tian, Z.R.; Su, O. Hexagonal and prismatic nanowalled ZnO microboxes. Inorg. Chem. 2006, 45, 3256–3260. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Hu, Y.L.; Pelligra, C.; Chen, C.H.; Jin, L.; Huang, H.; Sithambaram, S.; Aindow, M.; Joesten, R.; Suib, S.L. ZnO with Different Morphologies Synthesized by Solvothermal Methods for Enhanced Photocatalytic Activity. Chem. Mater. 2009, 21, 2875–2885. [Google Scholar] [CrossRef]
- Chang, J.; Waclawik, E.R. Facet-controlled self-assembly of ZnO nanocrystals by non-hydrolytic aminolysis and their photodegradation activities. CrystEngComm 2012, 14, 4041–4048. [Google Scholar] [CrossRef] [Green Version]
- Boppella, R.; Anjaneyulu, K.; Basak, P.; Manorama, S.V. Facile Synthesis of Face Oriented ZnO Crystals: Tunable Polar Facets and Shape Induced Enhanced Photocatalytic Performance. J. Phys. Chem. C 2013, 117, 4597–4605. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K. Zinc oxide based photocatalysis: Tailoring surface-bulk structure and related interfacial charge carrier dynamics for better environmental applications. RSC Adv. 2015, 5, 3306–3351. [Google Scholar] [CrossRef]
- Vallejos, S.; Pizurova, N.; Gracia, I.; Sotelo-Vazquez, C.; Cechal, J.; Blackman, C.; Parkin, I.; Cane, C. ZnO Rods with Exposed {100} Facets Grown via a Self-Catalyzed Vapor-Solid Mechanism and Their Photocatalytic and Gas Sensing Properties. ACS Appl. Mater. Interfaces 2016, 8, 33335–33342. [Google Scholar] [CrossRef] [PubMed]
- Scarano, D.; Cesano, F.; Bertarione, S.; Zecchina, A. Zinc Oxide Nanostructures: From Chestnut Husk-Like Structures to Hollow Nanocages, Synthesis and Structure. Crystals 2018, 8, 153. [Google Scholar] [CrossRef] [Green Version]
- Roza, L.; Fauzia, V.; Abd Rahman, M.Y. Tailoring the active surface sites of ZnO nanorods on the glass substrate for photocatalytic activity enhancement. Surf. Interfaces 2019, 15, 117–124. [Google Scholar] [CrossRef]
- Alshehri, N.A.; Lewis, A.R.; Pleydell-Pearce, C.; Maffeis, T.G.G. Investigation of the growth parameters of hydrothermal ZnO nanowires for scale up applications. J. Saudi Chem. Soc. 2018, 22, 538–545. [Google Scholar] [CrossRef] [Green Version]
- Kammel, R.S.; Sabry, R.S. Effects of the aspect ratio of ZnO nanorods on the performance of piezoelectric nanogenerators. J. Sci. Adv. Mater. Devices. 2019, 4, 420–424. [Google Scholar] [CrossRef]
- Heinonen, S.; Nikkanen, J.P.; Hakola, H.; Huttunen-Saarivirta, E.; Kannisto, M.; Hyvärinen, L.; Järveläinen, M.; Levänen, E. Effect of temperature and concentration of precursors on morphology and photocatalytic activity of zinc oxide thin films prepared by hydrothermal route. IOP Conf. Ser. Mater. Sci. Eng. 2016, 123, 012030. [Google Scholar] [CrossRef] [Green Version]
- van Embden, J.; Gross, S.; Kittilstved, K.R.; Della Gaspera, E. Colloidal Approaches to Zinc Oxide Nanocrystals. Chem. Rev. 2023, 123, 271–326. [Google Scholar] [CrossRef]
- Boucher, A.; Jones, G.; Roldan, A. Toward a new definition of surface energy for late transition metals. Phys. Chem. Chem. Phys. 2023, 25, 1977–1986. [Google Scholar] [CrossRef]
- Tyson, W.R.; Miller, W.A. Surface Free-Energies of Solid Metals—Estimation From Liquid Surface-Tension Measurements. Surf. Sci. 1977, 62, 267–276. [Google Scholar] [CrossRef]
- Wulff, G. XXVI. Zur Theorie des Krystallhabitus. Z. Kristallogr. Cryst. Mater. 1908, 45, 433–472. [Google Scholar] [CrossRef]
- Andrés, J.; Gracia, L.; Gouveia, A.F.; Ferrer, M.M.; Longo, E. Effects of surface stability on the morphological transformation of metals and metal oxides as investigated by first-principles calculations. Nanotechnology 2015, 26, 405703–405713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouveia, A.F.; Assis, M.; Cavalcante, L.S.; Gracia, L.; Longo, E.; Andres, J. Reading at exposed surfaces: Theoretical insights into photocatalytic activity of ZnWO4. Front. Res. Today 2018, 1, 1005. [Google Scholar] [CrossRef]
- Pereira, P.F.S.; Gouveia, A.F.; Assis, M.; de Oliveira, R.C.; Pinatti, I.M.; Penha, M.; Goncalves, R.F.; Gracia, L.; Andres, J.; Longo, E. ZnWO4 nanocrystals: Synthesis, morphology, photoluminescence and photocatalytic properties. Phys. Chem. Chem. Phys. 2018, 20, 1923–1937. [Google Scholar] [CrossRef]
- Gouveia, A.F.; Roca, R.A.; Macedo, N.G.; Cavalcante, L.S.; Longo, E.; San-Miguel, M.A.; Altomare, A.; da Silva, G.S.; Andrés, J. Ag2WO4 as a multifunctional material: Fundamentals and progress of an extraordinarily versatile semiconductor. J. Mater. Res. Technol. 2022, 21, 4023–4051. [Google Scholar] [CrossRef]
- Macedo, N.G.; Gouveia, A.F.; Roca, R.A.; Assis, M.; Gracia, L.; Andrés, J.; Leite, E.R.; Longo, E. Surfactant-Mediated Morphology and Photocatalytic Activity of α-Ag2WO4 Material. J. Phys. Chem. C 2018, 122, 8667–8679. [Google Scholar] [CrossRef]
- Roca, R.A.; Gouveia, A.F.; Lemos, P.S.; Gracia, L.; Andres, J.; Longo, E. Formation of Ag Nanoparticles on beta-Ag2WO4 through Electron Beam Irradiation: A Synergetic Computational and Experimental Study. Inorg. Chem. 2016, 55, 8661–8671. [Google Scholar] [CrossRef]
- Laier, L.O.; Assis, M.; Foggi, C.C.; Gouveia, A.F.; Vergani, C.E.; Santana, L.C.L.; Cavalcante, L.S.; Andres, J.; Longo, E. Surface-dependent properties of alpha-Ag2WO4: A joint experimental and theoretical investigation. Theor. Chem. Acc. 2020, 139, 108. [Google Scholar] [CrossRef]
- Ribeiro, R.A.P.; Oliveira, M.C.; Bomio, M.R.D.; de Lazaro, S.R.; Andres, J.; Longo, E. Connecting the surface structure, morphology and photocatalytic activity of Ag2O: An in depth and unified theoretical investigation. Appl. Surf. Sci. 2020, 509, 145321. [Google Scholar] [CrossRef]
- Ribeiro, R.A.P.; Andres, J.; Longo, E.; Lazaro, S.R. Magnetism and multiferroic properties at MnTiO3 surfaces: A DFT study. Appl. Surf. Sci. 2018, 452, 463–472. [Google Scholar] [CrossRef]
- Santiago, A.A.G.; Tranquilin, R.L.; Oliveira, M.C.; Ribeiro, R.A.P.; de Lazaro, S.R.; Correa, M.A.; Bohn, F.; Longo, E.; Motta, F.V.; Bomio, M.R.D. Disclosing the Structural, Electronic, Magnetic, and Morphological Properties of CuMnO2: A Unified Experimental and Theoretical Approach. J. Phys. Chem. C 2020, 124, 5378–5388. [Google Scholar] [CrossRef]
- Barbosa, M.D.; Fabris, G.D.L.; Ferrer, M.M.; de Azevedo, D.H.M.; Sambrano, J.R. Computational Simulations of Morphological Transformations by Surface Structures: The Case of Rutile TiO2 phase. Mater. Res. 2017, 20, 920–925. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, M.M.; Fabris, G.S.L.; de Faria, B.V.; Martins, J.B.L.; Moreira, M.L.; Sambrano, J.R. Quantitative evaluation of the surface stability and morphological changes of Cu2O particles. Heliyon 2019, 5, e02500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laranjeira, J.A.S.; Fabris, G.S.L.; Albuquerque, A.R.; Ferrer, M.M.; Sambrano, J.R. Morphological transformations mapping of CaXO4 (X = Mo or W) and their surface stability. Mater. Today Commun. 2022, 33, 104178. [Google Scholar] [CrossRef]
- La Porta, F.A.; Nogueira, A.E.; Gracia, L.; Pereira, W.S.; Botelho, G.; Mulinari, T.A.; Andres, J.; Longo, E. An experimental and theoretical investigation on the optical and photocatalytic properties of ZnS nanoparticles. J. Phys. Chem. Solids 2017, 103, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Tello, A.C.M.; Assis, M.; Menasce, R.; Gouveia, A.F.; Teodoro, V.; Jacomaci, N.; Zaghete, M.A.; Andres, J.; Marques, G.E.; Teodoro, M.D.; et al. Microwave-Driven Hexagonal-to-Monoclinic Transition in BiPO4: An In-Depth Experimental Investigation and First-Principles Study. Inorg. Chem. 2020, 59, 7453–7468. [Google Scholar] [CrossRef]
- Lacerda, L.H.d.S.; San-Miguel, M.A. DFT approaches unraveling the surface and morphological properties of MnMoO4. Appl. Surf. Sci. 2021, 567, 150882. [Google Scholar] [CrossRef]
- Laranjeira, J.A.S.; Ferrer, M.M.; Albuquerque, A.R.; Paskocimas, C.A.; Sambrano, J.R.; Fabris, G.S.L. Computational Simulations to Predict the Morphology of Nanostructures and Their Properties. In Research Topics in Bioactivity, Environment and Energy: Experimental and Theoretical Tools; Taft, C.A., de Lazaro, S.R., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 267–287. [Google Scholar]
- Martins, J.B.L.; Andrés, J.; Longo, E.; Taft, C.A. A theoretical study of (1010) and (0001) ZnO surfaces: Molecular cluster model, basis set and effective core potential dependence. J. Mol. Struct. THEOCHEM 1995, 330, 301–306. [Google Scholar] [CrossRef]
- Na, S.H.; Park, C.H. First-Principles Study of the Surface Energy and the Atom Cohesion of Wurtzite ZnO and ZnS—Implications for Nanostructure Formation. J. Korean Phys. Soc. 2010, 56, 498–502. [Google Scholar] [CrossRef]
- Ferrer, M.M.; Gouveia, A.F.; Gracia, L.; Longo, E.; Andres, J. A 3D platform for the morphology modulation of materials: First principles calculations on the thermodynamic stability and surface structure of metal oxides: Co3O4, α-Fe2O3, and In2O3. Model. Simul. Mater. Sci. Eng. 2016, 24, 025007–025016. [Google Scholar] [CrossRef]
- Gouveia, A.F.; Ferrer, M.M.; Sambrano, J.R.; Andres, J.; Longo, E. Modeling the atomic-scale structure, stability, and morphological transformations in the tetragonal phase of LaVO4. Chem. Phys. Lett. 2016, 660, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Barmparis, G.D.; Lodziana, Z.; Lopez, N.; Remediakis, I.N. Nanoparticle shapes by using Wulff constructions and first-principles calculations. Beilstein J. Nanotechnol. 2015, 6, 361–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beltrán, A.; Andrés, J.; Longo, E.; Leite, E.R. Thermodynamic argument about SnO2 nanoribbon growth. Appl. Phys. Lett. 2003, 83, 635–637. [Google Scholar] [CrossRef]
- Zhang, H.; Penn, R.L.; Lin, Z.; Cölfen, H. Nanocrystal growth via oriented attachment. CrystEngComm 2014, 16, 1407–1408. [Google Scholar] [CrossRef] [Green Version]
- Kirchner, H.O.K. Coarsening of grain-boundary precipitates. Metall. Mater. Trans. B 1971, 2, 2861–2864. [Google Scholar] [CrossRef]
- Xue, X.; Penn, R.L.; Leite, E.R.; Huang, F.; Lin, Z. Crystal growth by oriented attachment: Kinetic models and control factors. CrystEngComm 2014, 16, 1419–1429. [Google Scholar] [CrossRef]
- Wander, A.; Harrison, N.M. An ab initio study of ZnO. Surf. Sci. 2000, 457, L342–L346. [Google Scholar] [CrossRef]
- Wander, A.; Harrison, N.M. An ab-initio study of ZnO. Surf. Sci. 2000, 468, L851–L855. [Google Scholar]
- Wander, A.; Schedin, F.; Steadman, P.; Norris, A.; McGrath, R.; Turner, T.S.; Thornton, G.; Harrison, N.M. Stability of Polar Oxide Surfaces. Phys. Rev. Lett. 2001, 86, 3811–3814. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, Y.; Tse, K.; Deng, B.; Xu, H.; Zhu, J. New approaches for calculating absolute surface energies of wurtzite (0001)/(0001¯): A study of ZnO and GaN. J. Appl. Phys. 2016, 119, 205302. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Hong, Y.J.; Yoo, J.; Yi, G.-C.; Park, G.-S.; Kong, K.-J.; Chang, H. Surface morphology and growth mechanism of catalyst-free ZnO and Mgx Zn1−xO nanorods. Phys. Status Solidi Rapid Res. Lett. 2008, 2, 197–199. [Google Scholar] [CrossRef]
- Cooke, D.J.; Marmier, A.; Parker, S.C. Surface Structure of and Surfaces of ZnO with Density Functional Theory and Atomistic Simulation. J. Phys. Chem. B 2006, 110, 7985–7991. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Yang, X.; Zhao, D.; Zhang, L. First-principles study of adsorption mechanism of NH3 on different ZnO surfaces on organics photocatalytic degradation purpose. Comput. Mater. Sci. 2018, 141, 133–140. [Google Scholar] [CrossRef]
- Kiomarsipour, N.; Shoja Razavi, R. Characterization and optical property of ZnO nano-, submicro- and microrods synthesized by hydrothermal method on a large-scale. Superlattices Microstruct. 2012, 52, 704–710. [Google Scholar] [CrossRef]
- Amin, G.; Asif, M.H.; Zainelabdin, A.; Zaman, S.; Nur, O.; Willander, M. Influence of pH, Precursor Concentration, Growth Time, and Temperature on the Morphology of ZnO Nanostructures Grown by the Hydrothermal Method. J. Nanomater. 2011, 2011, 5. [Google Scholar] [CrossRef] [Green Version]
- Lemos, S.C.S.; Rezende, T.K.d.L.; Assis, M.; Romeiro, F.d.C.; Peixoto, D.A.; Gomes, E.d.O.; Jacobsen, G.M.; Teodoro, M.D.; Gracia, L.; Ferrari, J.L.; et al. Efficient Ni and Fe doping process in ZnO with enhanced photocatalytic activity: A theoretical and experimental investigation. Mater. Res. Bull. 2022, 152, 111849. [Google Scholar] [CrossRef]
- Wu, K.; Jia, Z.; Zhou, L.; Yuan, S.; Cui, J. Study on the effect of methanol on the morphology and optical properties of ZnO. Optik 2020, 205, 164250. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, X.; Zhang, D.; Qin, J.; Lu, C.; Ding, H.; Yan, X.; Tang, H.; Wang, M.; Zhang, Q. Facile morphology-controlled hydrothermal synthesis of flower-like self-organized ZnO architectures. Cryst. Res. Technol. 2011, 46, 1189–1194. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, D.; Liu, H.; Li, T.; Wang, J. ZnO Tetrakaidecahedrons with Coexposed {001}, {101}, and {100} Facets: Shape-Selective Synthesis and Enhancing Photocatalytic Performance. Cryst. Growth Des. 2019, 19, 2758–2764. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, J.; Xue, Y.; Yu, P.; Zhang, B.; Wang, L.; Liu, R. Effect of aspect ratio and surface defects on the photocatalytic activity of ZnO nanorods. Sci. Rep. 2014, 4, 4596. [Google Scholar] [CrossRef] [Green Version]
- Hezam, A.; Drmosh, Q.A.; Ponnamma, D.; Bajiri, M.A.; Qamar, M.; Namratha, K.; Zare, M.; Nayan, M.B.; Onaizi, S.A.; Byrappa, K. Strategies to Enhance ZnO Photocatalyst’s Performance for Water Treatment: A Comprehensive Review. Chem. Rec. 2022, 22, e202100299. [Google Scholar] [CrossRef]
- Mishra, R.K.; Kumar, V.; Trung, L.G.; Choi, G.J.; Ryu, J.W.; Bhardwaj, R.; Kumar, P.; Singh, J.; Lee, S.H.; Gwag, J.S. Recent advances in ZnO nanostructure as a gas-sensing element for an acetone sensor: A short review. Luminescence 2022, 1–15. [Google Scholar] [CrossRef]
- Cho, S.; Jang, J.-W.; Jung, S.-H.; Lee, B.R.; Oh, E.; Lee, K.-H. Precursor Effects of Citric Acid and Citrates on ZnO Crystal Formation. Langmuir 2009, 25, 3825–3831. [Google Scholar] [CrossRef]
- Lu, H.; Wang, S.; Zhao, L.; Li, J.; Dong, B.; Xu, Z. Hierarchical ZnO microarchitectures assembled by ultrathin nanosheets: Hydrothermal synthesis and enhanced photocatalytic activity. J. Mater. Chem. 2011, 21, 4228–4234. [Google Scholar] [CrossRef]
- Shrok, A. ZnO nanowires growth direction and parameters affecting their surface morphology. In Nanowires; Simas, R., Ed.; IntechOpen: Rijeka, Croatia, 2021; Chapter 4. [Google Scholar]
- Zhao, X.; Nagashima, K.; Zhang, G.; Hosomi, T.; Yoshida, H.; Akihiro, Y.; Kanai, M.; Mizukami, W.; Zhu, Z.; Takahashi, T.; et al. Synthesis of Monodispersedly Sized ZnO Nanowires from Randomly Sized Seeds. Nano Lett. 2020, 20, 599–605. [Google Scholar] [CrossRef]
- Kumar, S.; Sahare, P.D.; Kumar, S. Optimization of the CVD parameters for ZnO nanorods growth: Its photoluminescence and field emission properties. Mater. Res. Bull. 2018, 105, 237–245. [Google Scholar] [CrossRef]
- Ahmed, F.; Arshi, N.; Anwar, M.S.; Danish, R.; Koo, B.H. Morphological evolution of ZnO nanostructures and their aspect ratio-induced enhancement in photocatalytic properties. RSC Adv. 2014, 4, 29249–29263. [Google Scholar] [CrossRef]
- Liu, S.; Liu, C.R. Morphology Control by Pulsed Laser in Chemical Deposition Illustrated in ZnO Crystal Growth. Cryst. Growth Des. 2019, 19, 2912–2918. [Google Scholar] [CrossRef]






| (100) | (110) | (001) | (102) | (101) | (112) | (111) | Functional | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| 1.12 | 1.06 | – | 2.07 | 1.73 | 2.18 | 2.22 | 2.04 | LDA + U | [51] |
| 2.05 | 1.16 | 2.00 | – | – | – | – | – | B3LYP | [59,60,61] |
| 0.87 | – | – | – | 2.00 | – | – | 1.39 | PBE-D3 | [3] |
| 0.82 | – | 2.37 | – | – | – | – | 1.01 | PBE | [62] |
| 0.91 | 1.64 | 1.74 | 1.58 | GGA | [63] | ||||
| 1.19 | 1.23 | LDA | [64] | ||||||
| 0.680 3.796 | – | 0.897 8.671 * | 2.292 2.481 2.389 | – | – | – | – | PBE | [65] |
| A\B | |||||||
|---|---|---|---|---|---|---|---|
| – | 30.00° ΔE = − 0.96 | 90.00° ΔE = − 2.51 | 40.37° ΔE = − 2.68 | 53.98° ΔE = − 2.96 | 17.33° ΔE = − 2.16 | 31.97° ΔE = − 1.28 | |
| 30.00° ΔE = − 0.89 | – | 90.00° ΔE = − 2.54 | 28.39° ΔE = − 2.84 | 47.23° ΔE = − 3.18 | 34.24° ΔE = − 3.28 | 42.72° ΔE = − 1.84 | |
| 90.00° ΔE = − 1.97 | 90.00° ΔE = − 2.03 | – | 61.61° ΔE = − 1.04 | 42.77° ΔE = − 1.36 | 72.67° ΔE = − 4.09 | 58.03° ΔE = − 1.99 | |
| 40.37° ΔE = − 2.60 | 28.39° ΔE = − 2.84 | 61.61° ΔE = − 1.45 | – | 18.84° ΔE = − 0.34 | 29.67° ΔE = − 2.53 | 26.09° ΔE = − 1.19 | |
| 53.98° ΔE = − 2.80 | 47.23° ΔE = − 3.18 | 42.77° ΔE = − 1.32 | 18.84° ΔE = 0.34 | – | 38.73° ΔE = − 1.16 | 27.43° ΔE = − 3.56 | |
| 17.33° ΔE = − 0.94 | 34.24° ΔE = − 3.23 | 72.77° ΔE = − 4.08 | 29.67° ΔE = − 2.12 | 38.73° ΔE = − 0.94 | – | 14.64° ΔE = − 3.25 | |
| 31.97° ΔE = 0.79 | 42.72° ΔE = − 0.45 | 58.03° ΔE = − 1.84 | 26.09° ΔE = − 0.48 | 27.43° ΔE = − 3.52 | 14.64° ΔE = − 3.27 | – |
| Side Surfaces | Growth Directions | |
|---|---|---|
| < 0 | < 0\– | |
| < 0 | < 0 | |
| < 0 | < 0 | |
| < 0\– | < 0 | |
| < 0 | < 0 | |
| A\B | |||
|---|---|---|---|
| (Starting morphology) 42.77° = − 1.36 | (Starting morphology) 42.77° = − 1.32 | (a) 90.00° = − 1.75 | (a) 90.00° = − 2.42 |
18.84° = 0.34 | 18.84° = − 0.34 | (b) 28.39° = − 1.80 | (b) 28.39° = − 1.80 |
28.39° = − 2.84 | 28.39° = − 2.84 | ||
40.37° = − 2.60 | 40.37° = − 2.68 | (c) 28.39° = − 1.26 | (c) 28.39° = − 1.27 |
30.00° = − 0.89 | 30.00° = − 0.96 | ||
| A\B | |||
|---|---|---|---|
| (a) 90.00° = −1.22 | (a) 90.00° = −1.23 | (c) 61.61° ΔE = −0.61 | (c) 61.61° ΔE = −0.55 |
28.39° = −1.73 | 28.39° = −1.73 | ||
| (b) 61.61° = −0.78 | (b) 61.61° = −0.49 | (d) 61.61° = −0.51 | (d) 61.61° = −0.58 |
28.39° = −1.90 | 28.39° = −1.90 | 28.39° = −1.63 | 28.39° = −1.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouveia, A.F.; Lemos, S.C.S.; Leite, E.R.; Longo, E.; Andrés, J. Back to the Basics: Probing the Role of Surfaces in the Experimentally Observed Morphological Evolution of ZnO. Nanomaterials 2023, 13, 978. https://0-doi-org.brum.beds.ac.uk/10.3390/nano13060978
Gouveia AF, Lemos SCS, Leite ER, Longo E, Andrés J. Back to the Basics: Probing the Role of Surfaces in the Experimentally Observed Morphological Evolution of ZnO. Nanomaterials. 2023; 13(6):978. https://0-doi-org.brum.beds.ac.uk/10.3390/nano13060978
Chicago/Turabian StyleGouveia, Amanda F., Samantha C. S. Lemos, Edson R. Leite, Elson Longo, and Juan Andrés. 2023. "Back to the Basics: Probing the Role of Surfaces in the Experimentally Observed Morphological Evolution of ZnO" Nanomaterials 13, no. 6: 978. https://0-doi-org.brum.beds.ac.uk/10.3390/nano13060978