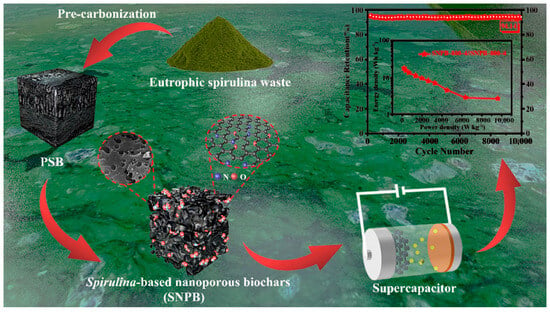
In Situ N, O-Dually Doped Nanoporous Biochar Derived from Waste Eutrophic Spirulina for High-Performance Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Structure and Surface Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
3.1. Material Characteristics
3.2. Electrochemical Properties in a Three-Electrode System
3.3. Electrochemical Properties in a Two-Electrode System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anto, S.; Mukherjee, S.S.; Muthappa, R.; Mathimani, T.; Deviram, G.; Kumar, S.S.; Verma, T.N.; Pugazhendhi, A. Algae as green energy reserve: Technological outlook on biofuel production. Chemosphere 2020, 242, 125079. [Google Scholar] [CrossRef] [PubMed]
- Sharuddin, S.D.A.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Lamba, P.; Singh, P.; Singh, P.; Singh, P.; Bharti; Kumar, A.; Gupta, M.; Kumar, Y. Recent advancements in supercapacitors based on different electrode materials: Classifications, synthesis methods and comparative performance. J. Energy Storage 2022, 48, 103871. [Google Scholar] [CrossRef]
- Loganathan, N.N.; Perumal, V.; Pandian, B.R.; Atchudan, R.; Edison, T.N.J.I.; Ovinis, M. Recent studies on polymeric materials for supercapacitor development. J. Energy Storage 2022, 49, 104149. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Han, Y.; Li, T. Flexible supercapacitor: Overview and outlooks. J. Energy Storage 2021, 42, 103053. [Google Scholar] [CrossRef]
- Hou, B.; Jin, X.; Jiang, L.; Li, Y.; Qiu, C.; Han, D.; Ding, Y.; Sheng, L. Flexible porous Graphene/Nickel hydroxide composite films with 3D ion transport channels for high volumetric performance asymmetric supercapacitor. Appl. Surf. Sci. 2021, 569, 151036. [Google Scholar] [CrossRef]
- Najib, S.; Erdem, E. Current progress achieved in novel materials for supercapacitor electrodes: Mini review. Nanoscale Adv. 2019, 1, 2817–2827. [Google Scholar] [CrossRef]
- Thanh Tam, L.T.; Tung, D.T.; Nguyet, H.M.; Ngoc Linh, N.T.; Dung, N.T.; Van Quynh, N.; Van Dang, N.; Vernardou, D.; Le, T.K.; Tuan, L.A.; et al. High electrochemical performance of ink solution based on manganese cobalt sulfide/reduced graphene oxide nano-composites for supercapacitor electrode materials. RSC Adv. 2022, 12, 20182–20190. [Google Scholar] [CrossRef]
- Dong, Y.-D.; Zhang, H.; Zhong, G.-J.; Yao, G.; Lai, B. Cellulose/carbon Composites and their Applications in Water Treatment—A Review. Chem. Eng. J. 2021, 405, 126980. [Google Scholar] [CrossRef]
- Shin, D.-Y.; Sung, K.-W.; Ahn, H.-J. Synergistic effect of heteroatom-doped activated carbon for ultrafast charge storage kinetics. Appl. Surf. Sci. 2019, 478, 499–504. [Google Scholar] [CrossRef]
- Lei, E.; Li, W.; Ma, C.; Xu, Z.; Liu, S. CO2-activated porous self-templated N-doped carbon aerogel derived from banana for high-performance supercapacitors. Appl. Surf. Sci. 2018, 457, 477–486. [Google Scholar]
- Shang, M.; Zhang, J.; Liu, X.; Liu, Y.; Guo, S.; Yu, S.; Filatov, S.; Yi, X. N, S self-doped hollow-sphere porous carbon derived from puffball spores for high performance supercapacitors. Appl. Surf. Sci. 2021, 542, 148697. [Google Scholar] [CrossRef]
- Sahin, M.E.; Blaabjerg, F.; Sangwongwanich, A. A Comprehensive Review on Supercapacitor Applications and Developments. Energies 2022, 15, 674. [Google Scholar] [CrossRef]
- Tafete, G.A.; Abera, M.K.; Thothadri, G. Review on nanocellulose-based materials for supercapacitors applications. J. Energy Storage 2022, 48, 103938. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, X.-L.; Yang, Y.-L.; Suo, G.; Zhang, L.; Lu, S.; Chen, Z.-G. Biomass-Derived Carbon for High-Performance Batteries: From Structure to Properties. Adv. Funct. Mater. 2022, 32, 2201584. [Google Scholar] [CrossRef]
- Ma, G.; Yang, Q.; Sun, K.; Peng, H.; Ran, F.; Zhao, X.; Lei, Z. Nitrogen-doped porous carbon derived from biomass waste for high-performance supercapacitor. Bioresour. Technol. 2015, 197, 137–142. [Google Scholar] [CrossRef]
- Ge, L.; Wu, Y.; Wang, F.; Huang, Y. Algae-Derived Nitrogen Self-Doped Porous Carbon Materials with High Supercapacitor Performances. Energy Fuels 2021, 35, 15118–15125. [Google Scholar] [CrossRef]
- Sun, J.; Norouzi, O.; Masek, O. A state-of-the-art review on algae pyrolysis for bioenergy and biochar production. Bioresour. Technol. 2022, 346, 126258. [Google Scholar] [CrossRef]
- Luo, M.; Wang, L.; Li, H.; Bu, Y.; Zhao, Y.; Cai, J. Hierarchical porous biochar from kelp: Insight into self-template effect and highly efficient removal of methylene blue from water. Bioresour. Technol. 2023, 372, 128676. [Google Scholar] [CrossRef]
- Zhu, S.J.; Gruschwitz, M.; Tsikourkitoudi, V.; Fischer, D.; Simon, F.; Tegenkamp, C.; Sommer, M.; Choudhury, S. All-Carbon Monolithic Composites from Carbon Foam and Hierarchical Porous Carbon for Energy Storage. ACS Appl. Mater. Interfaces 2022, 14, 44772–44781. [Google Scholar] [CrossRef]
- Yardim, Y.; Saka, C. Oxygen and nitrogen-doped carbon particles derived from pyrolysis of Chlorella vulgaris and Spirulina platensis microalgae as an efficient electrode material for supercapacitor application. Fuller. Nanotub. Carbon Nanostruct. 2023, 31, 713–723. [Google Scholar] [CrossRef]
- Okonkwo, C.A.; Lv, T.; Hong, W.; Li, G.; Huang, J.; Deng, J.; Jia, L.; Wu, M.; Liu, H.; Guo, M. The synthesis of micromesoporous carbon derived from nitrogen-rich spirulina extract impregnated castor shell based on biomass self-doping for highly efficient supercapacitor electrodes. J. Alloys Compd. 2020, 825, 154009. [Google Scholar] [CrossRef]
- Elma Karakaş, D.; Akdemir, M.; Atabani, A.E.; Kaya, M. A dual functional material: Spirulina Platensis waste-supported Pd-Co catalyst as a novel promising supercapacitor electrode. Fuel 2021, 304, 121334. [Google Scholar] [CrossRef]
- Bograchev, D.A.; Gryzlov, D.Y.; Sosenkin, V.E.; Volfkovich, Y.M. Modeling and experimental verification of operation of supercapacitors with carbon electrodes in non-aqueous electrolytes. The energy efficiency. Electrochim. Acta 2019, 319, 552–560. [Google Scholar] [CrossRef]
- Nie, W.; Cheng, H.; Liu, X.; Sun, Q.; Tian, F.; Yao, W.; Liang, S.; Lu, X.; Zhou, J. Surface organic nitrogen-doping disordered biomass carbon materials with superior cycle stability in the sodium-ion batteries. J. Power Sources 2022, 522, 230994. [Google Scholar] [CrossRef]
- Pourhosseini, S.E.M.; Norouzi, O.; Salimi, P.; Naderi, H.R. Synthesis of a Novel Interconnected 3D Pore Network Algal Biochar Constituting Iron Nanoparticles Derived from a Harmful Marine Biomass as High-Performance Asymmetric Supercapacitor Electrodes. Acs Sustain. Chem. Eng. 2018, 6, 4746–4758. [Google Scholar] [CrossRef]
- Song, M.; Zhou, Y.; Ren, X.; Wan, J.; Du, Y.; Wu, G.; Ma, F. Biowaste-based porous carbon for supercapacitor: The influence of preparation processes on structure and performance. J. Colloid Interface Sci. 2019, 535, 276–286. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, N.; Lei, S.; Yan, R.; Tian, X.; Wang, J.; Song, Y.; Xu, D.; Guo, Q.; Liu, L. Promising biomass-based activated carbons derived from willow catkins for high performance supercapacitors. Electrochim. Acta 2015, 166, 1–11. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, Z.; Guo, Y.; Guo, D.; Liu, G. Block copolymer derived uniform mesopores enable ultrafast electron and ion transport at high mass loadings. Nat. Commun. 2019, 10, 675. [Google Scholar] [CrossRef]
- An, W.; He, P.; Che, Z.; Xiao, C.; Guo, E.; Pang, C.; He, X.; Ren, J.; Yuan, G.; Du, N.; et al. Scalable Synthesis of Pore-Rich Si/C@C Core-Shell-Structured Microspheres for Practical Long-Life Lithium-Ion Battery Anodes. Acs Appl. Mater. Interfaces 2022, 14, 10308–10318. [Google Scholar] [CrossRef]
- Wu, G.; Ma, Z.; Wu, X.; Zhu, X.; Man, Z.; Lu, W.; Xu, J. Interfacial Polymetallic Oxides and Hierarchical Porous Core-Shell Fibres for High Energy-Density Electrochemical Supercapacitors. Angew. Chem. Int. Ed. 2022, 61, e2022037. [Google Scholar]
- Choma, J.; Jagiello, J.; Jaroniec, M. Assessing the contribution of micropores and mesopores from nitrogen adsorption on nanoporous carbons: Application to pore size analysis. Carbon 2021, 183, 150–157. [Google Scholar] [CrossRef]
- Liew, R.K.; Azwar, E.; Yek, P.N.Y.; Lim, X.Y.; Cheng, C.K.; Ng, J.-H.; Jusoh, A.; Lam, W.H.; Ibrahim, M.D.; Ma, N.L.; et al. Microwave pyrolysis with KOH/NaOH mixture activation: A new approach to produce micro-mesoporous activated carbon for textile dye adsorption. Bioresour. Technol. 2018, 266, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, F.; Song, Y.; Li, Y. Revitalizing carbon supercapacitor electrodes with hierarchical porous structures. J. Mater. Chem. A 2017, 5, 17705–17733. [Google Scholar] [CrossRef]
- Song, Z.; Liu, X.; Sun, X.; Li, Y.; Nie, X.; Tang, W.; Yu, R.; Shui, J. Alginate-templated synthesis of CoFe/carbon fiber composite and the effect of hierarchically porous structure on electromagnetic wave absorption performance. Carbon 2019, 151, 36–45. [Google Scholar] [CrossRef]
- Li, J.; Michalkiewicz, B.; Min, J.; Ma, C.; Chen, X.; Gong, J.; Mijowska, E.; Tang, T. Selective preparation of biomass-derived porous carbon with controllable pore sizes toward highly efficient CO2 capture. Chem. Eng. J. 2019, 360, 250–259. [Google Scholar] [CrossRef]
- Liu, R.; Hou, L.; Yue, G.; Li, H.; Zhang, J.; Liu, J.; Miao, B.; Wang, N.; Bai, J.; Cui, Z.; et al. Progress of Fabrication and Applications of Electrospun Hierarchically Porous Nanofibers. Adv. Fiber Mater. 2022, 4, 604–630. [Google Scholar]
- He, B.; Liu, J.; Zhang, Y.; Zhang, S.; Wang, P.; Xu, H. Comparison of structured activated carbon and traditional adsorbents for purification of H-2. Sep. Purif. Technol. 2020, 239, 116529. [Google Scholar] [CrossRef]
- Zhao, Y.-Q.; Lu, M.; Tao, P.-Y.; Zhang, Y.-J.; Gong, X.-T.; Yang, Z.; Zhang, G.-Q.; Li, H.-L. Hierarchically porous and heteroatom doped carbon derived from tobacco rods for supercapacitors. J. Power Sources 2016, 307, 391–400. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Jin, M.; Zhang, Y.; Niu, Y.-B.; Gao, J.-C.; Li, C.M. Chemically Exfoliating Biomass into a Graphene-like Porous Active Carbon with Rational Pore Structure, Good Conductivity, and Large Surface Area for High-Performance Supercapacitors. Adv. Energy Mater. 2018, 8, 1702545. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, G.; Zhu, L.; Wang, S. Green Conversion of Microalgae into High-Performance Sponge-Like Nitrogen-Enriched Carbon. ChemElectroChem 2019, 6, 646–652. [Google Scholar] [CrossRef]
- Dervishi, E.; Ji, Z.; Htoon, H.; Sykora, M.; Doorn, S.K. Raman spectroscopy of bottom-up synthesized graphene quantum dots: Size and structure dependence. Nanoscale 2019, 11, 16571–16581. [Google Scholar] [CrossRef]
- Lopez-Diaz, D.; Lopez Holgado, M.; Garcia-Fierro, J.L.; Mercedes Velazquez, M. Evolution of the Raman Spectrum with the Chemical Composition of Graphene Oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Ling, P.; Zhang, X.; Xu, K.; He, L.; Wang, Y.; Su, S.; Hu, S.; Xiang, J. Raman spectroscopy of biochar from the pyrolysis of three typical Chinese biomasses: A novel method for rapidly evaluating the biochar property. Energy 2020, 202, 117644. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Akbarzadeh, A.; Chong, L.; Du, J.; Tahir, N.; Awasthi, M.K. Catalytic pyrolysis of lignocellulosic biomass for bio-oil production: A review. Chemosphere 2022, 297, 134181. [Google Scholar] [CrossRef]
- Yogalakshmi, K.N.; Devi, T.P.; Sivashanmugam, P.; Kavitha, S.; Kannah, R.Y.; Varjani, S.; AdishKumar, S.; Kumar, G.; Banu, J.R. Lignocellulosic biomass-based pyrolysis: A comprehensive review. Chemosphere 2022, 286, 131824. [Google Scholar]
- Thines, K.R.; Abdullah, E.C.; Mubarak, N.M.; Ruthiraan, M. Synthesis of magnetic biochar from agricultural waste biomass to enhancing route for waste water and polymer application: A review. Renew. Sustain. Energy Rev. 2017, 67, 257–276. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, K.; Jiang, K.; Zhang, F.; Zhu, G.; Xu, H. Progress of synthetic strategies and properties of heteroatoms-doped (N, P, S, O) carbon materials for supercapacitors. J. Energy Storage 2022, 56, 105995. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Y.; Dai, Y.; Han, X.; Xing, B.; Zhu, L.; Qiu, K.; Wang, S. A novel approach for preparing in-situ nitrogen doped carbon via pyrolysis of bean pulp for supercapacitors. Energy 2021, 216, 119227. [Google Scholar] [CrossRef]
- Dong, D.; Zhang, Y.; Xiao, Y.; Wang, T.; Wang, J.; Romero, C.E.; Pan, W.-p. High performance aqueous supercapacitor based on nitrogen-doped coal-based activated carbon electrode materials. J. Colloid Interface Sci. 2020, 580, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, K.; Wang, D.; Teng, Z.; Cao, Y.; Qi, J.; Li, M.; Wang, M. Fabrication of high performance structural N-doped hierarchical porous carbon for supercapacitor. Carbon 2020, 164, 42–50. [Google Scholar] [CrossRef]
- Xie, C.; Yang, S.; Xu, X.; Shi, J.-W.; Niu, C. Core-shell structured carbon nanotubes/N-doped carbon layer nanocomposites for supercapacitor electrodes. J. Nanoparticle Res. 2020, 22, 25. [Google Scholar] [CrossRef]
- Kang, Z.; Pang, Z.; Zi, Z.; Liu, B.; Zhai, H.; Bai, Y.; Zhu, Z. Bifunctional N-doped hierarchical porous carbon nanosheets derived from heterologously-expressed spidroin for supercapacitors and oxygen reduction reaction. Biomass Bioenergy 2022, 163, 106528. [Google Scholar] [CrossRef]
- Ali, G.A.M.; Supriya, S.; Chong, K.F.; Shaaban, E.R.; Algarni, H.; Maiyalagan, T.; Hegde, G. Superior supercapacitance behavior of oxygen self-doped carbon nanospheres: A conversion of Allium cepa peel to energy storage system. Biomass Convers. Biorefinery 2021, 11, 1311–1323. [Google Scholar] [CrossRef]
- Ren, M.; Jia, Z.; Tian, Z.; Lopez, D.; Cai, J.; Titirici, M.-M.; Jorge, A.B. High Performance N-Doped Carbon Electrodes Obtained via Hydrothermal Carbonization of Macroalgae for Supercapacitor Applications. Chemelectrochem 2018, 5, 2686–2693. [Google Scholar] [CrossRef]
- Han, J.; Lee, K.; Choi, M.S.; Park, H.S.; Kim, W.; Roh, K.C. Chlorella-derived activated carbon with hierarchical pore structure for energy storage materials and adsorbents. Carbon Lett. 2019, 29, 167–175. [Google Scholar] [CrossRef]
- Perez-Salcedo, K.Y.; Ruan, S.; Su, J.; Shi, X.; Kannan, A.M.; Escobar, B. Seaweed-derived KOH activated biocarbon for electrocatalytic oxygen reduction and supercapacitor applications. J. Porous Mater. 2020, 27, 959–969. [Google Scholar] [CrossRef]
- Divya, P.; Rajalakshmi, R. Facile synthesis of micro/mesoporous functional carbon from Turbinaria conoides seaweed for high performance of supercapacitors. In Proceedings of the 1st International Conference on Recent Advances in Materials and Manufacturing (ICRAMM), Belagavi, India, 20–21 November 2020. [Google Scholar]
- Zhang, Z.; Bi, L.; Tian, Q.; Gao, J.-S.; He, Y. Construction of nickel/cobalt encapsulated by Ndoped carbon nanotubes for high performance supercapacitors. Diam. Relat. Mater. 2023, 139, 110237. [Google Scholar] [CrossRef]
- Pu, J.; Wang, L.; Huang, J.; Chen, Q.; Jin, Y.; Chen, J. High-performance supercapacitors based on porous activated carbon derived from carbon dots by directly pyrolyzing industrial glucose. Diam. Relat. Mater. 2023, 132, 109684. [Google Scholar] [CrossRef]
- Xie, Q.; Bao, R.; Xie, C.; Zheng, A.; Wu, S.; Zhang, Y.; Zhang, R.; Zhao, P. Core-shell N-doped active carbon fiber@graphene composites for aqueous symmetric supercapacitors with high-energy and high-power density. J. Power Sources 2016, 317, 133–142. [Google Scholar] [CrossRef]
- Zheng, C.; Zhou, X.; Cao, H.; Wang, G.; Liu, Z. Synthesis of porous graphene/activated carbon composite with high packing density and large specific surface area for supercapacitor electrode material. J. Power Sources 2014, 258, 290–296. [Google Scholar] [CrossRef]







| Sample | SBET a | Smicro b | Smeso c | Vt d | Vmicro e | Vmeso f | Vmeso/Vt | Pore Size g |
|---|---|---|---|---|---|---|---|---|
| (m2 g−1) | (m2 g−1) | (m2 g−1) | (cm3 g−1) | (cm3 g−1) | (cm3 g−1) | % | (d, nm) | |
| PSB | 0.9027 | —— | 2.2235 | 0.0025 | 0.0002 | 0.0028 | —— | 4.772 |
| SNPB-700-4 | 2515.8 | 537.4 | 632.3 | 1.3606 | 0.2065 | 0.4775 | 35.09 | 3.021 |
| SNPB-800-1 | 2096.1 | 1676.1 | 327.8 | 1.2051 | 0.6969 | 0.4385 | 36.39 | 5.351 |
| SNPB-800-2 | 2255.6 | 209.8 | 875.3 | 1.3788 | 0.0302 | 0.7421 | 53.82 | 3.391 |
| SNPB-800-4 | 2923.7 | —— | 1559.1 | 1.8448 | —— | 1.2114 | 65.67 | 3.108 |
| SNPB-900-4 | 2178.3 | —— | 1710.9 | 2.0687 | —— | 1.8981 | 91.75 | 4.297 |
| Electrode Material | Specific Surface Area (m2 g−1) | Specific Capacitance (F g−1) | Current Density (A g−1) | Energy Density (Wh kg−1) | Number of Cycles | Cycle Stability (%) | References |
|---|---|---|---|---|---|---|---|
| SNPB-800-4 | 2923.7 | 348.5 | 1 | 15.6 | 10,000 | 94.14 | —— |
| Enteromorpha prolifera | 2000 | 200 | 1 | 7 | 10,000 | 96 | [56] |
| Kelp | 4425 | 277 | 0.1 | 8 | 20,000 | 92 | [19] |
| Chlorella vulgaris | 3516 | 142 | 1 | 8.9 | 2200 | 91.5 | [57] |
| Ascophyllum nodosum | 1493 | 207.3 | 0.5 | — | 2500 | 92.3 | [58] |
| Turbinaria conoides | 173.8 | 416 | 1 | 52 | 5000 | 85.3 | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, Y.; Wang, J.; Chen, X.; Wang, Q.; Zhang, S.; Tian, Y.; Liu, C.; Wang, L.; Wei, Z.; Cao, L.; et al. In Situ N, O-Dually Doped Nanoporous Biochar Derived from Waste Eutrophic Spirulina for High-Performance Supercapacitors. Nanomaterials 2023, 13, 2431. https://0-doi-org.brum.beds.ac.uk/10.3390/nano13172431
Geng Y, Wang J, Chen X, Wang Q, Zhang S, Tian Y, Liu C, Wang L, Wei Z, Cao L, et al. In Situ N, O-Dually Doped Nanoporous Biochar Derived from Waste Eutrophic Spirulina for High-Performance Supercapacitors. Nanomaterials. 2023; 13(17):2431. https://0-doi-org.brum.beds.ac.uk/10.3390/nano13172431
Chicago/Turabian StyleGeng, Yihao, Jieni Wang, Xuanyu Chen, Qizhao Wang, Shuqin Zhang, Yijun Tian, Chenxiao Liu, Lin Wang, Zhangdong Wei, Leichang Cao, and et al. 2023. "In Situ N, O-Dually Doped Nanoporous Biochar Derived from Waste Eutrophic Spirulina for High-Performance Supercapacitors" Nanomaterials 13, no. 17: 2431. https://0-doi-org.brum.beds.ac.uk/10.3390/nano13172431