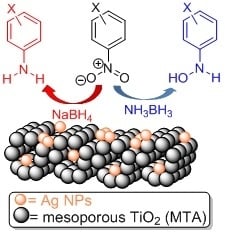
Reduction of Nitroarenes into Aryl Amines and N-Aryl hydroxylamines via Activation of NaBH4 and Ammonia-Borane Complexes by Ag/TiO2 Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Properties of Ag/TiO2 Catalysts
2.2. Evaluation of the Catalytic Activity
2.3. Chemoselective Reduction of Nitroarenes into Aryl Amines and N-Aryl Hydroxylamines
2.4. Kinetic Studies
3. Experimental Section
3.1. Materials
3.2. Synthesis of Ag/MTA Catalysts
3.3. Physical Characterization
3.4. Catalytic Reactions
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chaudhuri, R.G.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Sreeprasad, T.S.; Pradeep, T. Noble metal nanoparticles. In Springer Handbook of Nanomaterials; Vajtai, R., Ed.; Springer-Verlag: Berlin, Germany, 2013; pp. 303–388. [Google Scholar]
- Astruc, D. Transition-metal nanoparticles in catalysis: From historical background to the state-of-the art. In Nanoparticles and Catalysis; Astruc, D., Ed.; Wiley-VCH Verlag GmbH and Company KGaA: Weinheim, Germany, 2008; pp. 1–48. [Google Scholar]
- Doria, C.; Conde, J.; Veigas, B.; Giestas, L.; Almeida, C.; Assunção, M.; Rosa, J.; Baptista, P.V. Noble metal nanoparticles for biosensing applications. Sensor 2012, 12, 1657–1687. [Google Scholar] [CrossRef] [PubMed]
- De, M.; Ghosh, P.S.; Rotello, V.M. Applications of nanoparticles in biology. Adv. Mater. 2008, 20, 4225–4241. [Google Scholar] [CrossRef]
- El-Nour, K.M.M.A.; Eftaiha, A.; Al-Warthan, A.; Ammar, R.A.A. Synthesis and applications of silver nanoparticles. Arabian J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef]
- Tran, Q.H.; Nguyen, V.Q.; Le, A.-T. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4. [Google Scholar] [CrossRef]
- Bhosale, M.A.; Bhanage, B.M. Silver nanoparticles: Synthesis, characterization and their application as a sustainable catalyst for organic transformations. Curr. Org. Chem. 2015, 19, 708–727. [Google Scholar] [CrossRef]
- Abbiati, G.; Rossi, E. Silver and gold-catalyzed multicomponent reactions. Beilstein J. Org. Chem. 2014, 10, 481–513. [Google Scholar] [CrossRef] [PubMed]
- Rycnge, M.; Cobley, C.M.; Zeng, J.; Li, W.; Moran, C.H.; Zhang, Q.; Qin, D.; Xia, Y. Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem. Rev. 2011, 111, 3669–3712. [Google Scholar]
- Dong, X.-Y.; Gao, Z.-W.; Yang, K.-F.; Zhang, W.-Q.; Xu, L.-W. Nanosilver as a new generation of silver catalysts in organic transformations for efficient synthesis of fine chemicals. Catal. Sci. Technol. 2015, 5, 2554–2574. [Google Scholar] [CrossRef]
- Kundu, S.; Mandal, M.; Ghosh, S.K.; Pal, T. Photochemical deposition of SERS active silver nanoparticles on silica gel and their application as catalysts for the reduction of aromatic nitro compounds. J. Colloid Interface Sci. 2004, 272, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Davarpanah, J.; Kiasat, A.R. Catalytic application of silver nanoparticles immobilized to rice husk-SiO2-aminopropylsilane composite as recyclable catalyst in the aqueous reduction of nitroarenes. Catal. Commun. 2013, 41, 6–11. [Google Scholar] [CrossRef]
- Solanki, J.N.; Murthy, Z.V.P. Reduction of nitro aromatic compounds over Ag/Al2O3 nanocatalyst prepared in water-in-oil microemulsion: Effects of water-to-surfactant mole ratio and type of reducing agent. Ind. Eng. Chem. Res. 2011, 50, 7338–7344. [Google Scholar] [CrossRef]
- Zhou, Q.; Qian, G.; Li, Y.; Zhao, G.; Chao, Y.; Zheng, J. Two-dimensional assembly of silver nanoparticles for catalytic reduction of 4-nitroaniline. Thin Solid Films 2008, 516, 953–956. [Google Scholar] [CrossRef]
- Pradhan, N.; Pal, A.; Pal, T. Silver nanoparticle catalyzed reduction of aromatic nitro compounds. Colloids Surf. A 2002, 196, 247–257. [Google Scholar] [CrossRef]
- Dong, Z.; Le, X.; Li, X.; Zhang, W.; Dong, C.; Ma, J. Silver nanoparticles immobilized on fibrous nano-silica as highly efficient and recyclable heterogeneous catalyst for reduction of 4-nitrophenol and 2-nitroaniline. Appl. Catal. B 2014, 158, 129–135. [Google Scholar] [CrossRef]
- Chi, Y.; Tu, J.; Wang, M.; Li, X.; Zhao, Z. One-pot synthesis of ordered mesoporous silver nanoparticle/carbon composites for catalytic reduction of 4-nitrophenol. J. Colloid Interface Sci. 2014, 423, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.P.; Dhokale, R.K.; Yadav, H.M.; Achary, S.N.; Delekar, S.D. Titania-supported silver nanoparticles: An efficient and reusable catalyst for reduction of 4-nitrophenol. Appl. Surf. Sci. 2013, 273, 676–683. [Google Scholar] [CrossRef]
- Wang, M.; Tian, D.; Tian, P.; Yuan, L. Synthesis of micron-SiO2@nano-Ag particles and their catalyticperformance in 4-nitrophenol reduction. Appl. Surf. Sci. 2013, 283, 389–395. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Al-Sharif, M.S. One pot synthesis of silver nanoparticles supported on TiO2 using hybrid polymers as template and its efficient catalysis for the reduction of 4-nitrophenol. Mater. Chem. Phys. 2012, 136, 528–537. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Akita, T.; Ishida, T.; Haruta, M.; Xu, Q. Synergistic catalysis of Au@Ag core-shell nanoparticles stabilized on metal-organic framework. J. Am. Chem. Soc. 2011, 133, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Tamiolakis, I.; Fountoulaki, S.; Vordos, N.; Lykakis, I.N.; Armatas, G.S. Mesoporous Au-TiO2 nanoparticle assemblies as efficient catalysts for the chemoselective reduction of nitro compounds. J. Mater. Chem. A 2013, 1, 14311–14319. [Google Scholar] [CrossRef]
- Tamiolakis, I.; Lykakis, I.N.; Katsoulidis, A.P.; Armatas, G.S. One-pot synthesis of highly crystalline mesoporous TiO2 nanoparticle assemblies with enhanced photocatalytic activity. Chem. Commun. 2012, 48, 6687–6689. [Google Scholar] [CrossRef] [PubMed]
- Kamm, O. β-phenylhydroxylamines. Org. Synth. 1925, 4, 57–58. [Google Scholar]
- Nguyen-Tran, H.-H.; Zheng, G.-W.; Qian, X.-H.; Xu, J.-H. Highly selective and controllable synthesis of arylhydroxylamines by the reduction of nitroarenes with an electron-withdrawing group using a new nitroreductase BaNTR1. Chem. Commun. 2014, 50, 2861–2864. [Google Scholar] [CrossRef] [PubMed]
- Boymans, E.H.; Witte, P.T.; Vogt, D. A study on the selective hydrogenation of nitroaromatics to N-arylhydroxylamines using a supported Pt nanoparticle catalyst. Catal. Sci. Technol. 2015, 5, 176–183. [Google Scholar] [CrossRef]
- Rong, Z.; Du, W.; Wang, Y.; Lu, L. Carbon supported Pt colloid as effective catalyst for selective hydrogenation of nitroarenes to arylhydroxylamines. Chem. Commun. 2010, 46, 1559–1561. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, Y.; Kiyosu, T.; Choi, J.C.; Sakakura, T.; Yasuda, H. Selective synthesis of N-aryl hydroxylamines by the hydrogenation of nitroaromatics using supported platinum catalysts. Green Chem. 2009, 11, 1385–1390. [Google Scholar] [CrossRef]
- Pernoud, L.; Candy, P.; Didillon, B.; Jacquot, R.; Basset, J.M. Studies in Surface Science and Catalysis; Avelino Corma, F.V.M.S.M., José Luis, G.F., Eds.; Elsevier: Amsterdam, the Netherlands, 2000; Volume 130, pp. 2057–2062. [Google Scholar]
- Tamura, M.; Kon, K.; Satsuma, A.; Shimizu, K. Volcano-curves for dehydrogenation of 2-propanol and hydrogenation of nitrobenzene by SiO2-supported metal nanoparticles catalysts as described in terms of a d-band model. ACS Catal. 2012, 2, 1904–1909. [Google Scholar] [CrossRef]
- Widegren, J.A.; Finke, R.G. A review of soluble transition-metal nanoclusters as arene hydrogenation catalysts. J. Mol. Catal. A 2003, 191, 187–207. [Google Scholar] [CrossRef]
- Karwa, S.L.; Rajadhyaksha, R.A. Selective catalytic hydrogenation of nitrobenzene to phenylhydroxylamine. Ind. Eng. Chem. Res. 1987, 26, 1746–1750. [Google Scholar] [CrossRef]
- Shila, A.K.; Das, P. Solid supported platinum(0) nanoparticles catalyzed chemo-selective reduction of nitroarenes to N-arylhydroxylamines. Green Chem. 2013, 15, 3421–3428. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Jianga, J.; Jina, Z. The selective reduction of nitroarenes to N-arylhydroxylamines using Zn in a CO2/H2O system. Green Chem. 2009, 11, 1397–1400. [Google Scholar] [CrossRef]
- Ren, P.; Dong, T.; Wu, S. Synthesis of N-arylhydroxylamines by antimony-catalyzed reduction of nitroarenes. Synth. Commun. 1997, 27, 1547–1552. [Google Scholar] [CrossRef]
- Vasilikogiannaki, E.; Gryparis, C.; Kotzabasaki, V.; Lykakis, I.N.; Stratakis, M. Facile reduction of nitroarenes into anilines and nitroalkanes into hydroxylamines via the rapid activation of ammonia-borane complex by supported gold nanoparticles. Adv. Synth. Catal. 2013, 355, 907–911. [Google Scholar] [CrossRef]
- Lowry, T.H.; Richardson, K.S. Mechanism and Theory in Organic Chemistry, 3rd ed.; Harper & Row: New York, NY, USA, 1987; pp. 60–71. [Google Scholar]
- Fountoulaki, S.; Daikopoulou, V.; Gkizis, P.L.; Tamiolakis, I.; Armatas, G.S.; Lykakis, I.N. Mechanistic studies of the reduction of nitroarenes by NaBH4 or hydrosilanes catalyzed by supported gold nanoparticles. ACS Catal. 2014, 4, 3504–3511. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a theory of the van der Waals adsorption of gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Ravikovitch, P.I.; Wei, D.; Chueh, W.T.; Haller, G.L.; Neimark, A.V. Evaluation of pore structure parameters of MCM-41 catalyst supports and catalysts by means of nitrogen and argon adsorption. J. Phys. Chem. B 1997, 101, 3671–3679. [Google Scholar] [CrossRef]




| Entry | Catalyst 1 | Solvent | Reducing agent 2 | Time/Yield 3 |
|---|---|---|---|---|
| 1 | MTA or P25 | EtOH | NaBH4 | 24h/0% |
| 2 | 2% Ag/MTA | EtOH | NaBH4 | 4h/10% 4 |
| 3 | 3% Ag/MTA | EtOH | NaBH4 | 4h/51% 4 |
| 4 | 4% Ag/MTA | EtOH | NaBH4 | 4h/>99% |
| 5 | 7% Ag/MTA | EtOH | NaBH4 | 4h/30% 4 |
| 6 | AgOTf | EtOH | NaBH4 | 0.5h/>99% 5 |
| 7 | AgNO3 | EtOH | NaBH4 | 0.5h/>99%5 |
| 8 | Ag (wire) | EtOH | NaBH4 | 24h/0% |
| 9 | 4% Ag/MTA | EtOH | H2 (1 atm) | 24h/0% |
| 10 | 4% Ag/MTA | EtOH | TMDS | 24h/0% |
| 11 | 4% Ag/MTA | EtOH | DMPS | 4h/11% |
| 12 | 4% Ag/MTA | EtOH | NH2NH2·H2O | 24%/99% 6 |
| 13 | 4% Ag/MTA | EtOH | NH3BH3 | 0.2h/6% (94%) 7 |
| 14 | 4% Ag/MTA | MeOH | NaBH4 | 4h/99% |
| 15 | 4% Ag/MTA | Toluene | NaBH4 | 4h/0% |
| 16 | 4% Ag/MTA | THF | NaBH4 | 4h/0% |
| 17 | 4% Ag/MTA | CH3CN | NaBH4 | 4h/0% |
| 18 | 4% Ag/MTA | DCM | NaBH4 | 4h/0% |
| 19 | 4% Ag/MTA | Acetone | NaBH4 | 4h/0% |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreou, D.; Iordanidou, D.; Tamiolakis, I.; Armatas, G.S.; Lykakis, I.N. Reduction of Nitroarenes into Aryl Amines and N-Aryl hydroxylamines via Activation of NaBH4 and Ammonia-Borane Complexes by Ag/TiO2 Catalyst. Nanomaterials 2016, 6, 54. https://0-doi-org.brum.beds.ac.uk/10.3390/nano6030054
Andreou D, Iordanidou D, Tamiolakis I, Armatas GS, Lykakis IN. Reduction of Nitroarenes into Aryl Amines and N-Aryl hydroxylamines via Activation of NaBH4 and Ammonia-Borane Complexes by Ag/TiO2 Catalyst. Nanomaterials. 2016; 6(3):54. https://0-doi-org.brum.beds.ac.uk/10.3390/nano6030054
Chicago/Turabian StyleAndreou, Dimitrios, Domna Iordanidou, Ioannis Tamiolakis, Gerasimos S. Armatas, and Ioannis N. Lykakis. 2016. "Reduction of Nitroarenes into Aryl Amines and N-Aryl hydroxylamines via Activation of NaBH4 and Ammonia-Borane Complexes by Ag/TiO2 Catalyst" Nanomaterials 6, no. 3: 54. https://0-doi-org.brum.beds.ac.uk/10.3390/nano6030054