Improving Thermal, Mechanical, and Barrier Properties of Feather Keratin/Polyvinyl Alcohol/Tris(hydroxymethyl)aminomethane Nanocomposite Films by Incorporating Sodium Montmorillonite and TiO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of 1% Nanoparticle Solutions
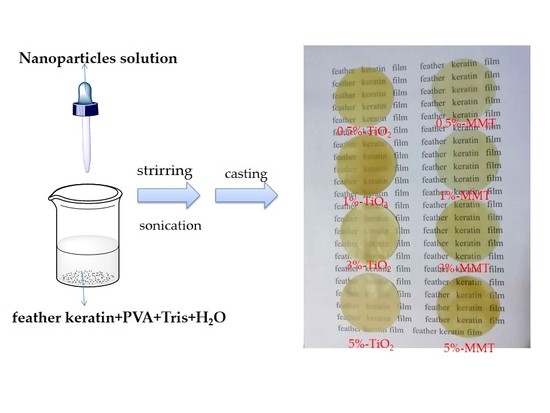
2.3. Preparation of the Nanocomposite Films
2.4. Characterization
3. Results and Discussion
3.1. Morphology of the Nanocomposite Films
3.2. FTIR Analysis
3.3. XRD Studies
3.4. TGA
3.5. Mechanical Properties
3.6. Barrier Properties of the Blend Films
3.6.1. WVP
3.6.2. Oxygen Permeability
3.6.3. Light Transmission and Transparency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abdorreza, M.N.; Cheng, L.; Karim, A. Effects of plasticizers on thermal properties and heat sealability of sago starch films. Food Hydrocoll. 2011, 25, 56–60. [Google Scholar] [CrossRef]
- Jang, S.A.; Shin, Y.J.; Song, K.B. Effect of rapeseed protein–gelatin film containing grapefruit seed extract on ‘maehyang’ strawberry quality. Int. J. Food Sci. Technol. 2011, 46, 620–625. [Google Scholar] [CrossRef]
- Jin, E.; Reddy, N.; Zhu, Z.; Yang, Y. Graft polymerization of native chicken feathers for thermoplastic applications. J. Agric. Food Chem. 2011, 59, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhou, H.; Hua, J. New progress in utilization of feather fibers. China Leather 2011, 40, 36–40. [Google Scholar]
- Zhang, Y.Q. Dissociation and Extraction of Feather Keratin by Steam Flash Explosion. Ph.D. Thesis, Jiangnan University, Wuxi, China, 2016. [Google Scholar]
- Brown, E.M.; Pandya, K.; Taylor, M.M.; Liu, C.-K. Comparison of methods for extraction of keratin from waste wool. Agric. Sci. 2016, 7, 670–679. [Google Scholar] [CrossRef]
- Shavandi, A.; Bekhit, A.E.; Carne, A.; Bekhit, A. Evaluation of keratin extraction from wool by chemical methods for bio-polymer application. J. Bioact. Compat. Polym. 2016, 32, 163–177. [Google Scholar] [CrossRef]
- Jiang, S.Q.; Zhang, L.; Wu, H.X.; Chi, G. Study on effective extraction of keratin from human hair wastes. Integr. Ferroelectr. 2017, 180, 102–107. [Google Scholar]
- Kang, D.; Herschend, J.; Al-Soud, W.A.; Mortensen, M.S.; Gonzalo, M.; Jacquiod, S.; Sørensen, S.J. Enrichment and characterization of an environmental microbial consortium displaying efficient keratinolytic activity. Bioresour. Technol. 2018, 270, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Sekar, V.; Kannan, M.; Ganesan, R.; Dheeba, B.; Sivakumar, N.; Kannan, K. Isolation and screening of keratinolytic bacteria from feather dumping soil in and around Cuddalore and Villupuram, Tamil Nadu. Proc. Natl. Acad. Sci. India 2015, 86, 1–9. [Google Scholar] [CrossRef]
- Dou, Y.; Huang, X.; Zhang, B.; He, M.; Yin, G.; Cui, Y. Preparation and characterization of dialdehyde starch crosslinked feather keratin film for food packaging application. RSC Adv. 2015, 5, 27168–27174. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, B.; He, M.; Yin, G.; Cui, Y.; Savina, I.N. Keratin/polyvinyl alcohol blend films cross-linked by dialdehyde starch and their potential application for drug release. Polymers 2015, 7, 580–591. [Google Scholar] [CrossRef]
- He, M.; Zhang, B.; Dou, Y.; Yin, G.; Cui, Y. Blend modification of feather keratin-based films using sodium alginate. J. Appl. Polym. Sci. 2017, 134, 44680–44687. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, B.N.; He, M.; Yin, G.Q.; Cui, Y.D. The structure, tensile properties and water resistance of hydrolyzed feather keratin-based bioplastics. Chin. J. Chem. Eng. 2016, 24, 415–420. [Google Scholar] [CrossRef]
- He, M.; Zhang, B.; Dou, Y.; Yin, G.; Cui, Y.; Chen, X. Fabrication and characterization of electrospun feather keratin/poly(vinyl alcohol) composite nanofibers. RSC Adv. 2017, 7, 9854–9861. [Google Scholar] [CrossRef] [Green Version]
- Isarankura Na Ayutthaya, S.; Tanpichai, S.; Wootthikanokkhan, J. Keratin extracted from chicken feather waste: Extraction, preparation, and structural characterization of the keratin and keratin/biopolymer films and electrospuns. J. Polym. Environ. 2015, 23, 506–516. [Google Scholar] [CrossRef]
- Sow, W.T.; Lui, Y.S.; Ng, K.W. Electrospun human keratin matrices as templates for tissue regeneration. Nanomedicine 2013, 8, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, S.; Yi, M.; Ge, J.; Yin, G.; Li, X. Preparation and physicochemical properties of blend films of feather keratin and poly(vinyl alcohol) compatibilized by tris(hydroxymethyl)aminomethane. Polymers 2018, 10, 1054. [Google Scholar] [CrossRef]
- Wu, S.; Chen, X.; Yi, M.; Ge, J.; Yin, G.; Li, X. Improving the water resistance and mechanical properties of feather keratin/polyvinyl alcohol/tris (hydroxymethyl) aminomethane blend films by cross-linking with transglutaminase, CaCl2, and genipin. Materials 2018, 11, 2203. [Google Scholar] [CrossRef]
- Oleyaei, S.A.; Almasi, H. Nanostructured materials utilized in biopolymer-based plastics for food packaging applications au-ghanbarzadeh, babak. Crit. Rev. Food Sci. Nutr. 2015, 55, 1699–1723. [Google Scholar]
- Abdallah, W.; Mirzadeh, A.; Tan, V.; Kamal, R.M. Influence of nanoparticle pretreatment on the thermal, rheological and mechanical properties of pla-pbsa nanocomposites incorporating cellulose nanocrystals or montmorillonite. Nanomaterials 2018, 9, 29. [Google Scholar] [CrossRef]
- Song, N.-B.; Jo, W.-S.; Song, H.-Y.; Chung, K.-S.; Won, M.; Song, K.B. Effects of plasticizers and nano-clay content on the physical properties of chicken feather protein composite films. Food Hydrocoll. 2013, 31, 340–345. [Google Scholar] [CrossRef]
- Bott, J.; Franz, R. Investigation into the potential migration of nanoparticles from laponite-polymer nanocomposites. Nanomaterials 2018, 8, 723. [Google Scholar] [CrossRef]
- El-Wakil, N.A.; Hassan, E.A.; Abou-Zeid, R.E.; Dufresne, A. Development of wheat gluten/nanocellulose/titanium dioxide nanocomposites for active food packaging. Carbohydr. Polym. 2015, 124, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.M.; Chang, H.H.; Chang, K.C.; Liu, Y.L.; Tseng, C.C. A comparative study of the bactericidal effect of photocatalytic oxidation by TIO2 on antibiotic-resistant and antibiotic-sensitive bacteria. J. Chem. Technol. Biotechnol. 2010, 85, 1642–1653. [Google Scholar] [CrossRef]
- Oleyaei, S.A.; Almasi, H.; Ghanbarzadeh, B.; Moayedi, A.A. Synergistic reinforcing effect of TIO2 and montmorillonite on potato starch nanocomposite films: Thermal, mechanical and barrier properties. Carbohydr. Polym. 2016, 152, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Zapata, P.A.; Zenteno, A.; Amigó, N.; Rabagliati, F.M.; Sepúlveda, F.; Catalina, F.; Corrales, T. Study on the photodegradation of nanocomposites based on polypropylene and TIO2 nanotubes. Polym. Degrad. Stab. 2016, 133, 101–107. [Google Scholar] [CrossRef]
- Soitong, T. Photo-degradation of polypropylene-ascorbic acid TIO2 composite films. Int. Polym. Proc. 2018, 33, 29–34. [Google Scholar] [CrossRef]
- Liu, M.; Inde, R.; Nishikawa, M.; Qiu, X.; Atarashi, D.; Sakai, E.; Nosaka, Y.; Hashimoto, K.; Miyauchi, M. Enhanced photoactivity with nanocluster-grafted titanium dioxide photocatalysts. ACS Nano 2014, 8, 7229–7238. [Google Scholar] [CrossRef]
- Mahlambi, M.M.; Ngila, C.J.; Mamba, B.B. Recent developments in environmental photocatalytic degradation of organic pollutants: The case of titanium dioxide nanoparticles—A review. J. Nanomater. 2015, 2015, 5. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Y.; Cai, X.; Wang, S. Fabrication of gelatin–TIO2 nanocomposite film and its structural, antibacterial and physical properties. Int. J. Biol. Macromol. 2016, 84, 153–160. [Google Scholar] [CrossRef]
- Ibrahim, S.M. Characterization, mechanical, and thermal properties of gamma irradiated starch films reinforced with mineral clay. J. Appl. Polym. Sci. 2011, 119, 685–692. [Google Scholar] [CrossRef]
- Deka, B.K.; Maji, T.K. Effect of TIO2 and nanoclay on the properties of wood polymer nanocomposite. Compos. Part A Appl. Sci. Manuf. 2011, 42, 2117–2125. [Google Scholar] [CrossRef]
- Almasi, H.; Ghanbarzadeh, B.; Entezami, A.A. Physicochemical properties of starch–cmc–nanoclay biodegradable films. Int. J. Biol. Macromol. 2010, 46, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Tang, X.; Alavi, S.; Faubion, J. Structure and physical properties of starch/poly vinyl alcohol/sodium montmorillonite nanocomposite films. J. Agric. Food. Chem. 2011, 59, 12384–12395. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, K.; Xiao, J.; Liu, Y.; Zhao, Y.; Liu, A. Performance of high amylose starch-composited gelatin films influenced by gelatinization and concentration. Int. J. Biol. Macromol. 2017, 94, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Hani, N.M.; Nirmal, N.P.; Fazial, F.F.; Mohtar, N.F.; Romli, S.R. Optical and thermo-mechanical properties of composite films based on fish gelatin/rice flour fabricated by casting technique. Prog. Org. Coat. 2015, 84, 115–127. [Google Scholar] [CrossRef]
- De Silva, R.T.; Mantilaka, M.; Ratnayake, S.P.; Amaratunga, G.A.J.; de Silva, K.M.N. Nano-mgo reinforced chitosan nanocomposites for high performance packaging applications with improved mechanical, thermal and barrier properties. Carbohydr. Polym. 2017, 157, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Bindu Sharmila, T.K.; Ayswarya, E.P.; Abraham, B.T.; Sabura Begum, P.M.; Thachil, E.T. Fabrication of partially exfoliated and disordered intercalated cloisite epoxy nanocomposites via in situ polymerization: Mechanical, dynamic mechanical, thermal and barrier properties. Appl. Clay Sci. 2014, 102, 220–230. [Google Scholar] [CrossRef]
- Nagarajan, M.; Benjakul, S.; Prodpran, T.; Songtipya, P. Characteristics of bio-nanocomposite films from tilapia skin gelatin incorporated with hydrophilic and hydrophobic nanoclays. J. Food Eng. 2014, 143, 195–204. [Google Scholar] [CrossRef]
- Goyat, M.S.; Rana, S.; Halder, S.; Ghosh, P.K. Facile fabrication of epoxy-TIO2 nanocomposites: A critical analysis of TIO2 impact on mechanical properties and toughening mechanisms. Ultrason. Sonochem. 2018, 40, 861–873. [Google Scholar] [CrossRef]
- Zhou, J.J.; Wang, S.Y.; Gunasekaran, S. Preparation and characterization of whey protein film incorporated with TIO2 nanoparticles. J. Food Sci. 2009, 74, N50–N56. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar]
- Ping, L.; Jun, P.Z.; Ya, L.M.; Kang, D.Y. Gelatin/montmorillonite hybrid nanocomposite. II. Swelling behavior. J. Appl. Polym. Sci. 2003, 88, 322–326. [Google Scholar]
- Bae, H.J.; Park, H.J.; Hong, S.I.; Byun, Y.J.; Darby, D.O.; Kimmel, R.M.; Whiteside, W.S. Effect of clay content, homogenization rpm, ph, and ultrasonication on mechanical and barrier properties of fish gelatin/montmorillonite nanocomposite films. LWT Food Sci. Technol. 2009, 42, 1179–1186. [Google Scholar] [CrossRef]
- El-Rehim, H.A.; Kamal, H.; Hegazy, E.-S.A.; Soliman, E.-S.; Sayed, A. Use of gamma rays to improve the mechanical and barrier properties of biodegradable cellulose acetate nanocomposite films. Radiat. Phys. Chem. 2018, 153, 180–187. [Google Scholar] [CrossRef]
- Mallakpour, S.; Barati, A. Efficient preparation of hybrid nanocomposite coatings based on poly(vinyl alcohol) and silane coupling agent modified TIO2 nanoparticles. Prog. Org. Coat. 2011, 71, 391–398. [Google Scholar] [CrossRef]
- Oleyaei, S.A.; Zahedi, Y.; Ghanbarzadeh, B.; Moayedi, A.A. Modification of physicochemical and thermal properties of starch films by incorporation of TIO2 nanoparticles. Int. J. Biol. Macromol. 2016, 89, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Zolfi, M.; Khodaiyan, F.; Mousavi, M.; Hashemi, M. The improvement of characteristics of biodegradable films made from kefiran–whey protein by nanoparticle incorporation. Carbohydr. Polym. 2014, 109, 118–125. [Google Scholar] [CrossRef]
- Farahnaky, A.; Dadfar, S.M.M.; Shahbazi, M. Physical and mechanical properties of gelatin–clay nanocomposite. J. Food Eng. 2014, 122, 78–83. [Google Scholar] [CrossRef]
- Hoque, M.S.; Benjakul, S.; Prodpran, T.; Songtipya, P. Properties of blend film based on cuttlefish (sepia pharaonis) skin gelatin and mungbean protein isolate. Int. J. Biol. Macromol. 2011, 49, 663–673. [Google Scholar] [CrossRef]
- Martucci, J.F.; Ruseckaite, R.A. Biodegradable bovine gelatin/na+-montmorillonite nanocomposite films. Structure, barrier and dynamic mechanical properties. Polym. Plast. Technol. Eng. 2010, 49, 581–588. [Google Scholar] [CrossRef]
- Nagarajan, M.; Benjakul, S.; Prodpran, T.; Songtipya, P. Properties of film from splendid squid (loligo formosana) skin gelatin with various extraction temperatures. Int. J. Biol. Macromol. 2012, 51, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Physico-chemical properties, morphology and antioxidant activity of film from fish skin gelatin incorporated with root essential oils. J. Food Eng. 2013, 117, 350–360. [Google Scholar] [CrossRef]
- Martucci, J.; Ruseckaite, R. Structure and properties of gelatin/montomorillonite nanocomposite films. In Recent Advances in Research on Biodegradable Polymers and Sustainable Polymers; Nova Publishers: Hauppauge, NY, USA, 2008; pp. 27–36. [Google Scholar]










| Sample | FK Powder (g) | 6% PVA (g) | 6% Tris (g) | 1% MMT (g) | 1% TiO2 (g) | H2O (g) |
|---|---|---|---|---|---|---|
| P-40-25 | 1.2 | 13.33 | 8.33 | 0 | 0 | 27.17 |
| 0.5%-MMT | 1.2 | 13.33 | 7.83 | 1 | 0 | 26.67 |
| 1%-MMT | 1.2 | 13.33 | 7.33 | 2 | 0 | 26.17 |
| 3%-MMT | 1.2 | 13.33 | 5.33 | 6 | 0 | 24.17 |
| 5%-MMT | 1.2 | 13.33 | 3.33 | 10 | 0 | 22.17 |
| 0.5%-TiO2 | 1.2 | 13.33 | 7.83 | 0 | 1 | 26.67 |
| 1%-TiO2 | 1.2 | 13.33 | 7.33 | 0 | 2 | 26.17 |
| 3%-TiO2 | 1.2 | 13.33 | 5.33 | 0 | 6 | 24.17 |
| 5%-TiO2 | 1.2 | 13.33 | 3.33 | 0 | 10 | 22.17 |
| Sample | Δ1 | Δ2 | Residue (%) | |
|---|---|---|---|---|
| Td1 (°C) | Tonset (°C) | Tmax (°C) | ||
| P-40-25 | 167.5 | 214.83 | 329.33 | 8.75 |
| 0.5%-MMT | 199.17 | 231.83 | 330.67 | 8.86 |
| 1%-MMT | 202.17 | 235.83 | 330.83 | 10.07 |
| 3%-MMT | 200.5 | 230.17 | 330.17 | 10.55 |
| 5%-MMT | 202.33 | 230 | 329.67 | 12.11 |
| 0.5%-TiO2 | 199.67 | 229.67 | 330.17 | 9.29 |
| 1%-TiO2 | 204 | 234.5 | 331 | 9.23 |
| 3%-TiO2 | 201.83 | 236.5 | 331.33 | 11.44 |
| 5%-TiO2 | 196.17 | 227.5 | 330 | 10.64 |
| Sample | %T | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 800 nm | 700 nm | 600 nm | 500 nm | 400 nm | 350 nm | 300 nm | 280 nm | 200 nm | |
| P-40-25 | 79.65 | 77.57 | 74.21 | 66.73 | 41.49 | 18.40 | 0.67 | 0.10 | 0.00 |
| 0.5%-MMT | 74.10 | 71.22 | 67.10 | 59.56 | 37.83 | 18.21 | 0.62 | 0.04 | 0.00 |
| 1%-MMT | 68.55 | 65.65 | 61.95 | 54.25 | 32.92 | 18.05 | 0.53 | 0.00 | 0.00 |
| 3%-MMT | 67.60 | 63.20 | 59.40 | 51.20 | 27.30 | 16.40 | 0.38 | 0.00 | 0.00 |
| 5%-MMT | 62.30 | 60.00 | 56.19 | 47.80 | 25.10 | 14.50 | 0.20 | 0.00 | 0.00 |
| 0.5%-TiO2 | 56.15 | 51.81 | 45.91 | 37.64 | 21.95 | 11.46 | 0.10 | 0.00 | 0.00 |
| 1%-TiO2 | 41.00 | 35.30 | 27.80 | 20.50 | 9.20 | 3.80 | 0.10 | 0.00 | 0.00 |
| 3%-TiO2 | 6.00 | 3.70 | 2.10 | 1.30 | 0.50 | 0.00 | 0.00 | 0.00 | 0.00 |
| 5%-TiO2 | 2.00 | 1.40 | 1.10 | 0.80 | 0.30 | 0.00 | 0.00 | 0.00 | 0.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Chen, X.; Yi, M.; Ge, J.; Yin, G.; Li, X.; He, M. Improving Thermal, Mechanical, and Barrier Properties of Feather Keratin/Polyvinyl Alcohol/Tris(hydroxymethyl)aminomethane Nanocomposite Films by Incorporating Sodium Montmorillonite and TiO2. Nanomaterials 2019, 9, 298. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9020298
Wu S, Chen X, Yi M, Ge J, Yin G, Li X, He M. Improving Thermal, Mechanical, and Barrier Properties of Feather Keratin/Polyvinyl Alcohol/Tris(hydroxymethyl)aminomethane Nanocomposite Films by Incorporating Sodium Montmorillonite and TiO2. Nanomaterials. 2019; 9(2):298. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9020298
Chicago/Turabian StyleWu, Shufang, Xunjun Chen, Minghao Yi, Jianfang Ge, Guoqiang Yin, Xinming Li, and Ming He. 2019. "Improving Thermal, Mechanical, and Barrier Properties of Feather Keratin/Polyvinyl Alcohol/Tris(hydroxymethyl)aminomethane Nanocomposite Films by Incorporating Sodium Montmorillonite and TiO2" Nanomaterials 9, no. 2: 298. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9020298