Effect of Acidity Levels and Feed Rate on the Porosity of Aerogel Extracted from Rice Husk under Ambient Pressure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis Process
2.2. Characterization Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lee, C.J.; Kim, G.S.; Hyun, S.H. Synthesis of silica aerogels from waterglass via new modified ambient drying. J. Mater. Sci. 2002, 37, 2237–2241. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Leventis, N.; Koebel, M.M. Aerogels Handbook; Springer Science & Business Media: Berlin, Germany, 2011. [Google Scholar]
- Sabri, F.; Boughter, J.D., Jr.; Gerth, D.; Skalli, O.; Phung, T.-C.N.; Tamula, G.-R.M.; Leventis, N. Histological evaluation of the biocompatibility of polyurea crosslinked silica aerogel implants in a rat model: A pilot study. PLoS ONE 2012, 7, e50686. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; García-González, C.A.; del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.W.; Baker, E.S.; Lynch, K.J.; Sabri, F. In vivo X-ray imaging of phosphor-doped PDMS and phosphor-doped aerogel biomaterials. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 823–830. [Google Scholar] [CrossRef]
- Power, M.; Hosticka, B.; Black, E.; Daitch, C.; Norris, P. Aerogels as biosensors: Viral particle detection by bacteria immobilized on large pore aerogel. J. Non-Cryst. Solids 2001, 285, 303–308. [Google Scholar] [CrossRef]
- Nayak, J.P.; Bera, J. Preparation of Silica Aerogel by Ambient Pressure Drying Process using Rice Husk Ash as Raw Material. Trans. Indian Ceram. Soc. 2009, 68, 91–94. [Google Scholar] [CrossRef]
- Tamai, K.; Ma, J.F. Characterization of silicon uptake by rice roots. New Phytol. 2003, 158, 431–436. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef]
- Mitani, N.; Ma, J.F. Uptake system of silicon in different plant species. J. Exp. Bot. 2005, 56, 1255–1261. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.F.; Tamai, K.; Yamaji, N.; Mitani, N.; Konishi, S.; Katsuhara, M.; Ishiguro, M.; Murata, Y.; Yano, M. A silicon transporter in rice. Nature 2006, 440, 688. [Google Scholar] [CrossRef]
- Tadjarodi, A.; Haghverdi, M.; Mohammadi, V. Preparation and characterization of nano-porous silica aerogel from rice husk ash by drying at atmospheric pressure. Mater. Res. Bull. 2012, 47, 2584–2589. [Google Scholar] [CrossRef]
- Rajanna, S.K.; Kumar, D.; Vinjamur, M.; Mukhopadhyay, M. Silica Aerogel Microparticles from Rice Husk Ash for Drug Delivery. Ind. Eng. Chem. Res. 2015, 54, 949–956. [Google Scholar] [CrossRef]
- James, J.; Rao, M.S. Silica from rice husk through thermal decomposition. Thermochim. Acta 1986, 97, 329–336. [Google Scholar] [CrossRef]
- Chakraverty, A.; Mishra, P.; Banerjee, H.D. Investigation of thermal decomposition of rice husk. Thermochim. Acta 1985, 94, 267–275. [Google Scholar] [CrossRef]
- Wang, W.; Martin, J.C.; Zhang, N.; Ma, C.; Han, A.; Sun, L. Harvesting silica nanoparticles from rice husks. J. Nanopart. Res. 2011, 13, 6981–6990. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, D.H.; Liang, D.T. In-Depth Investigation of Biomass Pyrolysis Based on Three Major Components: Hemicellulose, Cellulose and Lignin. Energy Fuels 2006, 20, 388–393. [Google Scholar] [CrossRef]
- Liou, T.-H.; Chang, F.-W.; Lo, J.-J. Pyrolysis Kinetics of Acid-Leached Rice Husk. Ind. Eng. Chem. Res. 1997, 36, 568–573. [Google Scholar] [CrossRef]
- Kalapathy, U.; Proctor, A.; Shultz, J. A simple method for production of pure silica from rice hull ash. Bioresour. Technol. 2000, 73, 257–262. [Google Scholar] [CrossRef]
- Haq, I.; Akhtar, K.; Malik, A. Effect of experimental variables on the extraction of silica from the rice husk ash. J. Chem. Soc. Pak. 2014, 36, 382–387. [Google Scholar]
- Hwang, S.-W.; Kim, T.-Y.; Hyun, S.-H. Effect of surface modification conditions on the synthesis of mesoporous crack-free silica aerogel monoliths from waterglass via ambient-drying. Microporous Mesoporous Mater. 2010, 130, 295–302. [Google Scholar] [CrossRef]
- Hwang, S.-W.; Kim, T.-Y.; Hyun, S.-H. Optimization of instantaneous solvent exchange/surface modification process for ambient synthesis of monolithic silica aerogels. J. Colloid Interface Sci. 2008, 322, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef]
- Aravind, P.; Warrier, K. Mixed Oxide Silica Aerogels Synthesised through Non-Supercritical Route for Functional Applications. Ph.D. Thesis, Cochin University of Science and Technology, Kerala, India, 2008. [Google Scholar]
- Lazaro, A.; van de Griend, M.; Brouwers, H.; Geus, J. The influence of process conditions and Ostwald ripening on the specific surface area of olivine nano-silica. Microporous Mesoporous Mater. 2013, 181, 254–261. [Google Scholar] [CrossRef]
- Sarawade, P.B.; Kim, J.-K.; Hilonga, A.; Kim, H.T. Production of low-density sodium silicate-based hydrophobic silica aerogel beads by a novel fast gelation process and ambient pressure drying process. Solid State Sci. 2010, 12, 911–918. [Google Scholar] [CrossRef]
- Hilonga, A.; Kim, J.-K.; Sarawade, P.B.; Kim, H.T. Low-density TEOS-based silica aerogels prepared at ambient pressure using isopropanol as the preparative solvent. J. Alloys Compd. 2009, 487, 744–750. [Google Scholar] [CrossRef]
- Rao, A.P.; Rao, A.V. Modifying the surface energy and hydrophobicity of the low-density silica aerogels through the use of combinations of surface-modification agents. J. Mater. Sci. 2010, 45, 51–63. [Google Scholar]
- Shewale, P.M.; Rao, A.V.; Rao, A.P. Effect of different trimethyl silylating agents on the hydrophobic and physical properties of silica aerogels. Appl. Surf. Sci. 2008, 254, 6902–6907. [Google Scholar] [CrossRef]









| Oxide | Mass % |
|---|---|
| SiO2 | 92.07 |
| K2O | 3.16 |
| C | 1.53 |
| CaO | 0.76 |
| P2O5 | 0.66 |
| SO3 | 0.56 |
| N | 0.27 |
| MgO | 0.24 |
| MnO | 0.21 |
| F | 0.20 |
| Others | 0.35 |
| Sample | Na (ppm) | Si (ppm) |
|---|---|---|
| Sodium silicate | 22,808.75 | 29,586.03 |
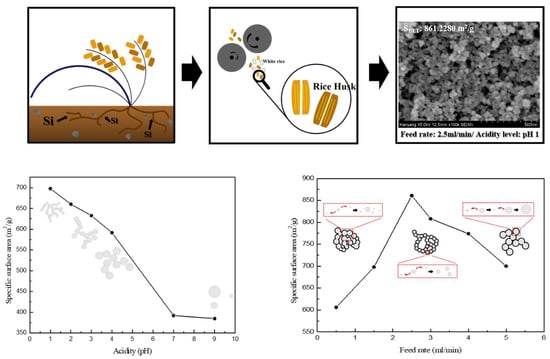
| pH | Specific Surface Area (m2/g) | Mean Pore Diameter (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| 1 | 697.7559 | 15.9180 | 2.7767 |
| 2 | 660.1251 | 17.6105 | 2.9062 |
| 3 | 632.4830 | 15.9664 | 2.5246 |
| 4 | 591.4873 | 18.6045 | 2.7510 |
| 7 | 391.9549 | 30.3362 | 2.9726 |
| 9 | 384.6501 | 28.5409 | 2.7445 |
| Feed Rate (mL/min) | Specific Surface Area (m2/g) | Mean Pore Diameter (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| 0.5 | 606.2524 | 15.8509 | 2.4024 |
| 1.5 | 697.7559 | 15.9180 | 2.7767 |
| 2.5 | 861.2280 | 15.6587 | 3.3714 |
| 3.0 | 808.4978 | 12.5028 | 2.5271 |
| 4.0 | 774.2045 | 13.1030 | 2.5361 |
| 5.0 | 700.5603 | 15.8025 | 2.7676 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ban, G.; Song, S.; Lee, H.W.; Kim, H.T. Effect of Acidity Levels and Feed Rate on the Porosity of Aerogel Extracted from Rice Husk under Ambient Pressure. Nanomaterials 2019, 9, 300. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9020300
Ban G, Song S, Lee HW, Kim HT. Effect of Acidity Levels and Feed Rate on the Porosity of Aerogel Extracted from Rice Husk under Ambient Pressure. Nanomaterials. 2019; 9(2):300. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9020300
Chicago/Turabian StyleBan, Garram, Sinae Song, Hong Woon Lee, and Hee Taik Kim. 2019. "Effect of Acidity Levels and Feed Rate on the Porosity of Aerogel Extracted from Rice Husk under Ambient Pressure" Nanomaterials 9, no. 2: 300. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9020300