Spiderweb-Like Fe-Co Prussian Blue Analogue Nanofibers as Efficient Catalyst for Bisphenol-A Degradation by Activating Peroxymonosulfate
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
2.2. Synthesis of (Prussian Blue Analogue) PBA Nanoparticles
2.3. Fabrication of Fe-Co Prussian Blue Analogue/Polyacrylonitrile (FCPBA/PAN) Nanofiber
2.4. Characterization
2.5. Catalytic Activity Measurements
3. Results and Discussion
3.1. Synthesis and Characterization of FCPBA/PAN
3.2. Activation of PMS by FCPBA/PAN
3.3. Effect of pH on the Degradation of BPA
3.4. Effect of Temperature on the Degradation of BPA
3.5. Effects of Catalyst Dosage, PMS Dosage and BPA Concentration
3.6. Effect of Anions and Organics
3.7. Possible Degradation Mechanism
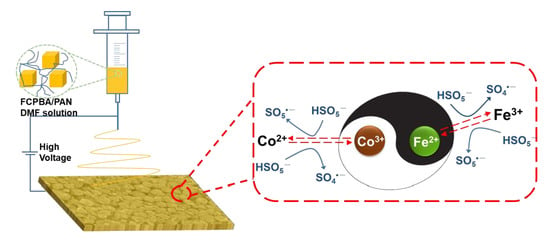
3.8. Synergistic Effect of Fe and Co
3.9. Reusability
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Liang, P.; Zhang, C.; Duan, X.; Sun, H.; Liu, S.; Tade, M.O.; Wang, S. An insight into metal organic framework derived N-doped graphene for the oxidative degradation of persistent contaminants: Formation mechanism and generation of singlet oxygen from peroxymonosulfate. Environ. Sci. Nano 2017, 4, 315–324. [Google Scholar] [CrossRef]
- Oh, W.D.; Dong, Z.; Lim, T.T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Hu, P.; Long, M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B Environ. 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Lai, B.; Yang, P. Insight into a highly efficient electrolysis-ozone process for N, N-dimethylacetamide degradation: Quantitative analysis of the role of catalytic ozonation, fenton-like and peroxone reactions. Water Res. 2018, 140, 12–23. [Google Scholar] [CrossRef]
- Hasan, Z.; Jhung, S.H. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef]
- Haque, E.; Jun, J.W.; Jhung, S.H. Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235). J. Hazard. Mater. 2011, 185, 507–511. [Google Scholar] [CrossRef]
- Bourgin, M.; Beck, B.; Boehler, M.; Borowska, E.; Fleiner, J.; Salhi, E.; Teichler, R.; Gunten, U.; Siegrist, H.; McArdell, C.S. Evaluation of a full-scale wastewater treatment plant upgraded with ozonation and biological post-treatments: Abatement of micropollutants, formation of transformation products and oxidation by-products. Water Res. 2018, 129, 486–498. [Google Scholar] [CrossRef]
- Grandclement, C.; Seyssiecq, I.; Piram, A.; Wong-Wah-Chung, P.; Vanot, G.; Tiliacos, N.; Roche, N.; Doumenq, P. From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: A review. Water Res. 2017, 111, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tian, J.; Zhu, Z.; Cui, F.; Zhu, Y.A.; Duan, X.; Wang, S. Magnetic nitrogen-doped nanocarbons for enhanced metal-free catalytic oxidation: Integrated experimental and theoretical investigations for mechanism and application. Chem. Eng. J. 2018, 354, 507–516. [Google Scholar] [CrossRef]
- Jaafarzadeh, N.; Ghanbari, F.; Ahmadi, M. Efficient degradation of 2,4-dichlorophenoxyacetic acid by peroxymonosulfate/magnetic copper ferrite nanoparticles/ozone: A novel combination of advanced oxidation processes. Chem. Eng. J. 2017, 320, 436–447. [Google Scholar] [CrossRef]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Removal of pharmaceuticals from water by homo/heterogonous Fenton-type processes—A review. Chemosphere 2017, 174, 665–688. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zhang, H.; Qian, Z.; Ouyang, T.; Sun, H.; Qin, J.; Tadé, M.O.; Wang, S. Bread-making synthesis of hierarchically Co@C nanoarchitecture in heteroatom doped porous carbons for oxidative degradation of emerging contaminants. Appl. Catal. B Environ. 2018, 225, 76–83. [Google Scholar] [CrossRef]
- Qi, F.; Chu, W. Heterogeneous catalytic ozonation of phenacetin in water using magnetic spinel ferrite as catalyst: Comparison of surface property and efficiency. J. Mol. Catal. A-Chem. 2015, 396, 164–173. [Google Scholar] [CrossRef]
- Qi, F.; Chu, W.; Xu, B. Modeling the heterogeneous peroxymonosulfate/Co-MCM41 process for the degradation of caffeine and the study of influence of cobalt sources. Chem. Eng. J. 2014, 235, 10–18. [Google Scholar] [CrossRef]
- Li, J.; Xu, M.; Yao, G.; Lai, B. Enhancement of the degradation of atrazine through CoFe2O4 activated peroxymonosulfate (PMS) process: Kinetic, degradation intermediates, and toxicity evaluation. Chem. Eng. J. 2018, 348, 1012–1024. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt. Environ. Sci. Technol. 2003, 37, 4790–4797. [Google Scholar] [CrossRef]
- Du, J.; Bao, J.; Liu, Y.; Ling, H.; Zheng, H.; Kim, S.H.; Dionysiou, D.D. Efficient activation of peroxymonosulfate by magnetic Mn-MGO for degradation of bisphenol A. J. Hazard. Mater. 2016, 320, 150–159. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, M.; Zhu, L. Activation of persulfate by Co3O4 nanoparticles for orange G degradation. RSC Adv. 2016, 6, 758–768. [Google Scholar] [CrossRef]
- Oh, W.D.; Dong, Z.; Hu, Z.T.; Lim, T.T. A novel quasi-cubic CuFe2O4–Fe2O3 catalyst prepared at low temperature for enhanced oxidation of bisphenol A via peroxymonosulfate activation. J. Mater. Chem. A 2015, 3, 22208–22217. [Google Scholar] [CrossRef]
- Li, H.; Wan, J.; Ma, Y.; Wang, Y.; Chen, X.; Guan, Z. Degradation of refractory dibutyl phthalate by peroxymonosulfate activated with novel catalysts cobalt metal-organic frameworks: Mechanism, performance, and stability. J. Hazard. Mater. 2016, 318, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Ban, Y.; Peng, Y.; Jin, H.; Bux, H.; Xu, L.; Caro, J.; Yang, W. Improvement of hydrothermal stability of zeolitic imidazolate frameworks. Chem. Commun. 2013, 49, 9140–9142. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.Y.A.; Chang, H.A. Zeolitic Imidazole Framework-67 (ZIF-67) as a heterogeneous catalyst to activate peroxymonosulfate for degradation of Rhodamine B in water. J. Taiwan Inst. Chem. Eng. 2015, 53, 40–45. [Google Scholar] [CrossRef]
- Lin, K.Y.A.; Chen, B.J.; Chen, C.K. Improvement of hydrothermal stability of zeolitic imidazolate frameworks. RSC Adv. 2016, 6, 92923–92933. [Google Scholar] [CrossRef]
- Zeng, L.; Xiao, L.; Shi, X.; Wei, M.; Cao, J.; Long, Y. Core-shell Prussian blue analogues@ poly(m-phenylenediamine) as efficient peroxymonosulfate activators for degradation of Rhodamine B with reduced metal leaching. J. Colloid. Interf. Sci. 2019, 534, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yao, J.; Xiao, P.; Shang, J.; Feng, Y.; Webley, P.A.; Wang, H. One-step fabrication of ZIF-8/polymer composite spheres by a phase inversion method for gas adsorption. Colloid Polym. Sci. 2013, 291, 2711–2717. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, F.; Zhang, S.; Cai, Y.; Cao, S.; Li, S.; Zhao, W.; Yuan, S.; Feng, X.; Cao, A.; et al. Facile Fabrication of Multifunctional Metal-Organic Framework Hollow Tubes to Trap Pollutants. J. Am. Chem. Soc. 2017, 139, 16482–16485. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Sutrisna, P.D.; Zhang, Y.; Chen, V. Continuous Metal-Organic Framework Membranes on Flexible Polymer Substrates. Angew. Chem. Int. Ed. 2016, 55, 3947–3951. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Nanofibrous metal–organic framework composite membrane for selective efficient oil/water emulsion separation. J. Membrane Sci. 2017, 543, 10–17. [Google Scholar] [CrossRef]
- Armstrong, M.R.; Shan, B.; Maringanti, S.V.; Zheng, W.; Mu, B. Hierarchical Pore Structures and High ZIF-8 Loading on Matrimid Electrospun Fibers by Additive Removal from a Blended Polymer Precursor. Ind. Eng. Chem. Res. 2016, 55, 9944–9951. [Google Scholar] [CrossRef]
- Lu, A.X.; McEntee, M.; Browe, M.A.; Hall, M.G.; DeCoste, J.B.; Peterson, G.W. MOFabric: Electrospun Nanofiber Mats from PVDF/UiO-66-NH2 for Chemical Protection and Decontamination. ACS Appl. Mater. Interfaces 2017, 9, 13632–13636. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, F.; Liu, H.; Zhu, W.; Teng, M.; Jiang, Y.; Li, W.; Xu, D.; He, D.; Hannam, P.; et al. Electrospun fibrous mats as skeletons to produce free-standing MOF membranes. J. Mater. Chem. 2012, 22, 16971. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Wang, X.; Yu, J.; Ding, B. Ultrahigh Metal-Organic Framework Loading and Flexible Nanofibrous Membranes for Efficient CO2 Capture with Long-Term, Ultrastable Recyclability. ACS Appl. Mater. Interfaces 2018, 10, 34802–34810. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, S.; Feng, X.; Li, H.; Zhou, J.; Wang, B. Preparation of Nanofibrous Metal-Organic Framework Filters for Efficient Air Pollution Control. J. Am. Chem. Soc. 2016, 138, 5785–5788. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, H.; Luo, R.; Liu, C.; Li, J.; Sun, X.; Shen, J.; Han, W.; Wang, L. Metal-organic framework one-dimensional fibers as efficient catalysts for activating peroxymonosulfate. Chem. Eng. J. 2017, 330, 262–271. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.; Lee, W.; Kim, S. Rapid removal of radioactive cesium by polyacrylonitrile nanofibers containing Prussian blue. J. Hazard. Mater. 2018, 347, 106–113. [Google Scholar] [CrossRef]
- Xi, J.; Xia, Y.; Xu, Y.; Xiao, J.; Wang, S. (Fe, Co)@nitrogen-doped graphitic carbon nanocubes derived from polydopamine-encapsulated metal-organic frameworks as a highly stable and selective non-precious oxygen reduction electrocatalyst. Chem. Commun. 2015, 51, 10479–10482. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Luo, R.; Liu, C.; Li, J.; Sun, X.; Shen, J.; Han, W.; Wang, L. Metal–organic framework derived Co3O4/C@SiO2 yolk–shell nanoreactors with enhanced catalytic performance. J. Mater. Chem. A 2018, 6, 11226–11235. [Google Scholar] [CrossRef]
- Luo, R.; Liu, C.; Li, J.; Wang, J.; Hu, X.; Sun, X.; Shen, J.; Han, W.; Wang, L. Nanostructured CoP: An efficient catalyst for degradation of organic pollutants by activating peroxymonosulfate. J. Hazard. Mater. 2017, 329, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Anipsitakis, G.P.; Dionysiou, D.D. Transition metal/UV-based advanced oxidation technologies for water decontamination. Appl. Catal. B Environ. 2004, 54, 155. [Google Scholar] [CrossRef]
- Xu, Y.; Ai, J.; Zhang, H. The mechanism of degradation of bisphenol A using the magnetically separable CuFe2O4/peroxymonosulfate heterogeneous oxidation process. J. Hazard. Mater. 2016, 309, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Mishra, I.M.; Dionysiou, D.D.; Kumar, V. Oxidative removal of Bisphenol A by UV-C/peroxymonosulfate (PMS): Kinetics, influence of co-existing chemicals and degradation pathway. Chem. Eng. J. 2015, 276, 193–204. [Google Scholar] [CrossRef]
- Du, Y.; Ma, W.; Liu, P.; Zou, B.; Ma, J. Magnetic CoFe2O4 nanoparticles supported on titanate nanotubes (CoFe2O4/TNTs) as a novel heterogeneous catalyst for peroxymonosulfate activation and degradation of organic pollutants. J. Hazard. Mater. 2016, 308, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Shi, Z.; Zou, Z.; Zhou, S. Magnetic EDTA functionalized CoFe2O4 nanoparticles (EDTA- CoFe2O4) as a novel catalyst for peroxymonosulfate activation and degradation of Orange G. Environ. Sci. Pollut. Res. Int. 2017, 24, 11536–11548. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Ji, C.; Zhong, L.; Liu, S.; Cui, F.; Sun, H.; Wang, W. Magnetic Fe–Co crystal doped hierarchical porous carbon fibers for removal of organic pollutants. J. Mater. Chem. A 2017, 5, 18071–18080. [Google Scholar] [CrossRef]
- Lin, K.Y.; Chang, H.A.; Chen, R.C. MOF-derived magnetic carbonaceous nanocomposite as a heterogeneous catalyst to activate oxone for decolorization of Rhodamine B in water. Chemosphere 2015, 130, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Dong, C.; Kong, D.; Lu, J. New insights into atrazine degradation by cobalt catalyzed peroxymonosulfate oxidation: Kinetics, reaction products and transformation mechanisms. J. Hazard. Mater. 2015, 285, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.X.; Wang, C.Y.; Yang, C.W.; Guo, P.C.; Yu, H.Q. Degradation of Bisphenol A by Peroxymonosulfate Catalytically Activated with Mn1.8Fe1.2O4 Nanospheres: Synergism between Mn and Fe. Environ. Sci. Technol. 2017, 51, 12611–12618. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Bao, H.; Yao, Y.; Lu, W.; Chen, W. Novel green activation processes and mechanism of peroxymonosulfate based on supported cobalt phthalocyanine catalyst. Appl. Catal. B Environ. 2014, 154–155, 36–43. [Google Scholar] [CrossRef]
- Gong, C.; Chen, F.; Yang, Q.; Luo, K.; Yao, F.; Wang, S.; Wang, X.; Wu, J.; Li, X.; Wang, D.; et al. Heterogeneous activation of peroxymonosulfate by Fe-Co layered doubled hydroxide for efficient catalytic degradation of Rhoadmine B. Chem. Eng. J. 2017, 321, 222–232. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, G.; Liu, M.; Wang, Q.; Wang, P. Optimization of the catalytic activity of a ZnCo2O4 catalyst in peroxymonosulfate activation for bisphenol A removal using response surface methodology. Chemosphere 2018, 212, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kim, J.; Malgras, V.; Na, J.; Lin, J.; You, J.; Zhang, M.; Li, J.; Yamauchi, Y. Metal-organic frameworks and their derived materials: Emerging catalysts for sulfate radicals-based advanced oxidation process in water purification. Small 2019. [Google Scholar] [CrossRef]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, C.; Qi, J.; Yan, Y.; Zhang, M.; Yan, X.; Sun, X.; Wang, L.; Li, J. Spiderweb-Like Fe-Co Prussian Blue Analogue Nanofibers as Efficient Catalyst for Bisphenol-A Degradation by Activating Peroxymonosulfate. Nanomaterials 2019, 9, 402. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9030402
Wang H, Wang C, Qi J, Yan Y, Zhang M, Yan X, Sun X, Wang L, Li J. Spiderweb-Like Fe-Co Prussian Blue Analogue Nanofibers as Efficient Catalyst for Bisphenol-A Degradation by Activating Peroxymonosulfate. Nanomaterials. 2019; 9(3):402. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9030402
Chicago/Turabian StyleWang, Hongyu, Chaohai Wang, Junwen Qi, Yubo Yan, Ming Zhang, Xin Yan, Xiuyun Sun, Lianjun Wang, and Jiansheng Li. 2019. "Spiderweb-Like Fe-Co Prussian Blue Analogue Nanofibers as Efficient Catalyst for Bisphenol-A Degradation by Activating Peroxymonosulfate" Nanomaterials 9, no. 3: 402. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9030402