Cholic Acid-Conjugated Methylcellulose-Polyethylenimine Nano-Aggregates for Drug Delivery Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of Cholic Acid-conjugated Methylcellulose-polyethylenimines (MCPEI-CAs)
2.3. Measurement of Critical Aggregation Concentration (CAC)
2.4. Encapsulation of Dox in MCPEI-CA Nano-aggregates
2.5. Evaluation of Dox Loading in Cholic Acid-conjugated Methylcellulose-polyethylenimines (MCPEI-CA)/Doxorubicin (Dox) Nano-aggregates
2.6. Average Particle Size and Zeta-Potential Measurements
2.7. Transmission Electron Microscopy (TEM)
2.8. Release Profile of Doxorubicin (Dox) from Cholic Acid-conjugated Methylcellulose-polyethylenimines (MCPEI-CA) Nano-aggregates
2.9. Circular Dichroism (CD) Measurements
2.10. Congo Red Absorbance Measurements
2.11. Cell Culture
2.12. MTT Assay
2.13. Lactate Dehydrogenase (LDH) Assay
2.14. Anticancer Activity of Cholic Acid-conjugated Methylcellulose-polyethylenimines (MCPEI-CA)/Doxorubicin (Dox) Nano-aggregates
3. Results and Discussion
3.1. Synthesis and Characterization of Cholic Acid-conjugated Methylcellulose-polyethylenimines (MCPEI-CAs)
3.2. Characterization of Cholic Acid-conjugated Methylcellulose-Polyethylenimines (MCPEI-CA) Nano-aggregates for Drug Delivery Systems
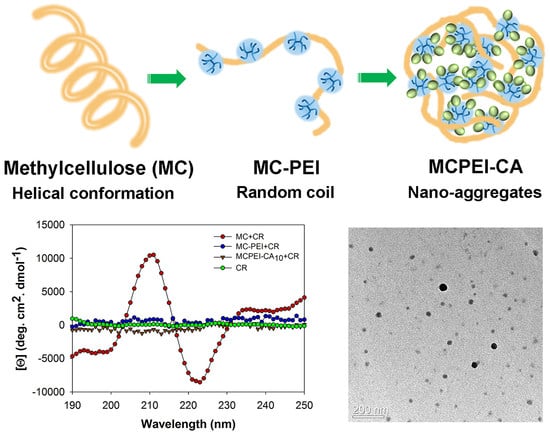
3.3. Molecular Conformation of Polymers
3.4. In vitro Cell Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Bao, G.; Mitragotri, S.; Tong, S. Multifunctional nanoparticles for drug delivery and molecular Imaging. Annu. Rev. Biomed. Eng. 2013, 15, 253–282. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S. Dendrimers as versatile platform in drug delivery applications. Eur. J. Pharm. Biopharm. 2009, 71, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Gai, S.; Lin, J. Functionalized mesoporous silica materials for controlled drug delivery. Chem. Soc. Rev. 2012, 41, 3679–3698. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Tabakman, S.M.; Yang, K.; Dai, H. Carbon materials for drug delivery & cancer therapy. Mater. Today 2011, 14, 316–323. [Google Scholar]
- Han, G.; Ghosh, P.; Rotello, V.M. Functionalized gold nanoparticles for drug delivery. Nanomedicine 2007, 2, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef]
- Oh, J.K.; Lee, D.I.; Park, J.M. Biopolymer-based microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2009, 34, 1261–1282. [Google Scholar] [CrossRef]
- Nitta, S.; Numata, K. Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. Int. J. Mol. Sci. 2013, 14, 1629–1654. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef]
- Dheer, D.; Arora, D.; Jaglan, S.; Rawal, R.K.; Shankar, R. Polysaccharides based nanomaterials for targeted anti-cancer drug delivery. J. Drug Target. 2017, 25, 1–16. [Google Scholar] [CrossRef]
- Hirrien, M.; Desbrières, J.; Rinaudo, M. Physical properties of methylcelluloses in relation with the conditions for cellulose modification. Carbohydr. Polym. 1996, 31, 243–252. [Google Scholar] [CrossRef]
- Nasatto, P.L.; Pignon, F.; Silveira, J.L.M.; Duarte, M.E.R.; Noseda, M.D.; Rinaudo, M. Methylcellulose, a cellulose derivative with original physical properties and extended applications. Polymers 2015, 7, 777–803. [Google Scholar] [CrossRef]
- Kim, K.; Ryu, K.; Kim, T.-i. Cationic methylcellulose derivative with serum-compatibility and endosome buffering ability for gene delivery systems. Carbohydr. Polym. 2014, 110, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.W. The enzymes, regulation, and genetics of bile Acid synthesis. Annu. Rev. Biochem. 2003, 72, 137–174. [Google Scholar] [CrossRef]
- Simonović, B.R.; Momirović, M. Determination of critical micelle concentration of bile acid salts by micro-calorimetric titration. Mikrochim. Acta 1997, 127, 101–104. [Google Scholar] [CrossRef]
- Chae, S.Y.; Kim, H.J.; Lee, M.S.; Jang, Y.L.; Lee, Y.; Lee, S.H.; Lee, K.; Kim, S.H.; Kim, H.T.; Chi, S.-C.; et al. Energy-independent intracellular gene delivery mediated by polymeric biomimetics of cell-penetrating peptides. Macromol. Biosci. 2011, 11, 1169–1174. [Google Scholar] [CrossRef]
- Amjad, M.W.; Amin, M.C.I.M.; Katas, H.; Butt, A.M. Doxorubicin-loaded cholic acid-polyethyleneimine micelles for targeted delivery of antitumor drugs: Synthesis, characterization, and evaluation of their in vitro cytotoxicity. Nanoscale Res. Lett. 2012, 7, 687. [Google Scholar] [CrossRef]
- Jia, Y.-G.; Zhu, X.X. Thermo- and pH-responsive copolymers bearing cholic acid and oligo(ethylene glycol) pendants: Self-assembly and pH-controlled release. ACS Appl. Mater. Interfaces 2015, 7, 24649–24655. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, C. Mechanism of hepatic targeting via oral administration of DSPE–PEG–cholic acid-modified nanoliposomes. Int. J. Nanomed. 2017, 12, 1673–1684. [Google Scholar] [CrossRef] [Green Version]
- Basu Ray, G.; Chakraborty, I.; Moulik, S.P. Pyrene absorption can be a convenient method for probing critical micellar concentration (cmc) and indexing micellar polarity. J. Colloid Interface Sci. 2006, 294, 248–254. [Google Scholar] [CrossRef]
- Ritcey, A.M.; Gray, D.G. Induced CD provides evidence for helical solution conformation in cellulosic chains. Biopolymers 1988, 27, 479–491. [Google Scholar] [CrossRef]
- Vold, I.M.N.; Christensen, B.E. Periodate oxidation of chitosans with different chemical compositions. Carbohydr. Res. 2005, 340, 679–684. [Google Scholar] [CrossRef]
- Zhou, B.; Zhao, L.; Shen, M.; Zhao, J.; Shi, X. A multifunctional polyethylenimine-based nanoplatform for targeted anticancer drug delivery to tumors in vivo. J. Mater. Chem. B 2017, 5, 1542–1550. [Google Scholar] [CrossRef]
- Allenmark, S. Induced circular dichroism by chiral molecular interaction. Chirality 2003, 15, 409–422. [Google Scholar] [CrossRef]
- Inouye, H.; Kirschner, D.A. Alzheimer’s β-amyloid: Insights into fibril formation and structure from Congo red binding. Subcell. Biochem. 2005, 38, 203–224. [Google Scholar]
- Deng, Y.; Yuan, W.; Jia, Z.; Liu, G. H- and J-Aggregation of fluorene-based chromophores. J. Phys. Chem. B 2014, 118, 14536–14545. [Google Scholar] [CrossRef]
- Dean, J.C.; Oblinsky, D.G.; Rafiq, S.; Scholes, G.D. Methylene blue exciton states steer nonradiative relaxation: Ultrafast spectroscopy of methylene blue dimer. J. Phys. Chem. B 2016, 120, 440–454. [Google Scholar] [CrossRef] [PubMed]
- Weyermann, J.; Lochmann, D.; Zimmer, A. A practical note on the use of cytotoxicity assays. Int. J. Pharm. 2005, 288, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Salomon, J.J.; Ehrhardt, C. Nanoparticles attenuate P-glycoprotein/MDR1 function in A549 human alveolar epithelial cells. Eur. J. Pharm. Biopharm. 2011, 77, 392–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, T.; Köhler, C.; Weidauer, E.; Taege, C.; Foth, H. Expression of MRP1 and related transporters in human lung cells in culture. Toxicology 2001, 167, 59–72. [Google Scholar] [CrossRef]
- Krishna, R.; Mayer, L.D. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 2000, 11, 265–283. [Google Scholar] [CrossRef]







| [PEI]:[CA] (Feed Ratio) | [PEI]:[CA] (1H NMR) | |
|---|---|---|
| MCPEI-CA3 | 1:3.6 | 1:1.74 |
| MCPEI-CA6 | 1:6.0 | 1:2.98 |
| MCPEI-CA10 | 1:10.0 | 1:3.65 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.; Park, J.; Kim, T.-i. Cholic Acid-Conjugated Methylcellulose-Polyethylenimine Nano-Aggregates for Drug Delivery Systems. Nanomaterials 2019, 9, 459. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9030459
Kim T, Park J, Kim T-i. Cholic Acid-Conjugated Methylcellulose-Polyethylenimine Nano-Aggregates for Drug Delivery Systems. Nanomaterials. 2019; 9(3):459. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9030459
Chicago/Turabian StyleKim, Taewan, Jaehong Park, and Tae-il Kim. 2019. "Cholic Acid-Conjugated Methylcellulose-Polyethylenimine Nano-Aggregates for Drug Delivery Systems" Nanomaterials 9, no. 3: 459. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9030459