Synthetic Biodegradable Aliphatic Polyester Nanocomposites Reinforced with Nanohydroxyapatite and/or Graphene Oxide for Bone Tissue Engineering Applications
Abstract
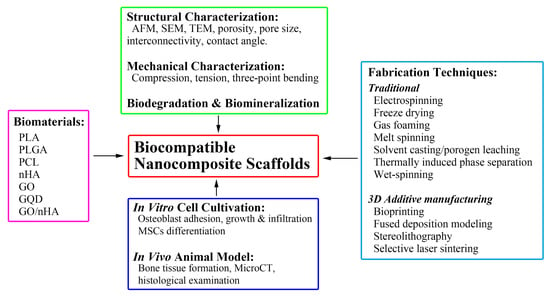
:1. Introduction
2. Preparation of Nanofillers
2.1. Nanohydroxyapatite
2.2. Graphene Oxide and Its Derivatives
2.3. Graphene Oxide-Nanohydroxyapatite
3. Preparation of Polymer Bionanocomposite Scaffolds
3.1. PLA-Based Nanocomposites
3.1.1. PLA-nHA Nanocomposites
Processing and Properties of PLA/nHA Nanocomposites
Processing and Properties of PLA/nHA Scaffolds
3.1.2. PLA/GO Nanocomposites
Processing and Properties of PLA/GO Nanocomposites
Processing and Properties of PLA/GO Scaffolds
3.1.3. PLA/nHA-GO Hybrid Nanocomposites
Processing and Properties of PLA/nHA-GO Hybrid Nanocomposites
Processing and Properties of Hybrid PLA/nHA-GO Scaffolds
3.2. PLGA-Based Nanocomposite Scaffolds
3.3. PCL-Based Nanocomposites
3.3.1. PCL/nHA Nanocomposites
Properties of PCL/nHA Nanocomposites
Structure and Properties of Porous PCL/nHA Scaffolds
3.3.2. PCL/GO Nanocomposites
Properties of PCL/GO Nanocomposites
Properties of Porous PCL/GO Scaffolds
3.3.3. PCL/nHA-GO Hybrid Nanocomposite
4. In Vitro Studies
4.1. In Vitro Hydrolytic Degradation
4.2. Biomineralization
4.3. In Vitro Cell Cultivation
4.3.1. Bionanocomposites
4.3.2. Bionanocomposite Scaffolds
Bionanocomposite Scaffolds with nHA Fillers
Bionanocomposite Scaffolds with GO Fillers
5. In Vivo Animal Models
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yu, X.; Tang, X.; Gohil, S.V.; Laurencin, C.T. Biomaterials for bone regenerative engineering. Adv. Healthc. Mater. 2015, 24, 1268–1285. [Google Scholar] [CrossRef] [PubMed]
- Sozen, T.; Ozisik, L.; Basaran, N.C. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Lems, W.F.; Raterman, H.G. Critical issues and current challenges in osteoporosis and fracture prevention. An overview of unmet needs. Ther. Adv. Musculoskelet. Dis. 2017, 9, 299–316. [Google Scholar] [CrossRef] [Green Version]
- Guise, T.A. Bone loss and fracture risk associated with cancer therapy. Oncologist 2006, 11, 1121–1131. [Google Scholar] [CrossRef]
- Ibrahim, T.; Mercatali, L.; Amadon, D. Bone and cancer: The osteoncology. Clin. Cases Miner. Bone Metab. 2013, 10, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Valery, P.C.; Laversanne, M.; Bray, F. Bone cancer incidence by morphological subtype: A global assessment. Cancer Causes Control 2015, 26, 1127–1139. [Google Scholar] [CrossRef]
- Wascher, D.C.; Bulthuis, L. Extremity trauma: Field management of sports injuries. Curr. Rev. Musculoskelet. Med. 2014, 7, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef]
- Schwartz, A.M.; Shenker, M.L.; Ahn, J.; Willett, N.J. Building better bone: The weaving of biologic and engineering strategies for managing bone loss. J. Orthop. Res. 2017, 35, 1855–1864. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babaie, E.; Bhaduri, S.B. Fabrication aspects of porous biomaterials in orthopedic applications: A review. ACS Biomater. Sci. Eng. 2018, 4, 1–39. [Google Scholar] [CrossRef]
- Chen, X.; Fan, H.; Deng, X.; Wu, L.; Yi, T.; Gu, L.; Zhou, C.; Fan, Y.; Zhang, X. Scaffold structural microenvironmental cues to guide tissue regeneration in bone tissue applications. Nanomaterials 2018, 8, 960. [Google Scholar] [CrossRef]
- Neto, A.S.; Ferreira, J.M. Synthetic and marine-derived porous scaffolds for bone tissue engineering. Materials 2018, 11, 1702. [Google Scholar] [CrossRef] [PubMed]
- Bruzauskate, I.; Bironaite, D.; Bagdonas, E.; Bernotiene, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—Different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef]
- Goldstein, S.A. The mechanical properties of trabecular bone: Dependence on anatomic location and function. J. Biomech. 1987, 20, 1055–1061. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef]
- Wu, S.; Xiao, Z.; Song, J.; Li, M.; Li, W. Evaluation of BMP-2 enhances the osteoblast differentiation of human amnion mesenchymal stem cells seeded on nano-hydroxyapatite/collagen/poly(l-lactide). Int. J. Mol. Sci. 2018, 19, 2171. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic materials: Current challenges and opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Tjong, S.C.; Yeager, E. ESCA and SIMS studies of the passive film on iron. J. Electrochem. Soc. 1981, 128, 2251–2254. [Google Scholar] [CrossRef]
- Tjong, S.C.; Hoffman, R.W.; Yeager, E.B. Electron and ion spectroscopic studies of the passive film on iron-chromium alloys. J. Electrochem. Soc. 1982, 129, 1662–1668. [Google Scholar] [CrossRef]
- Brooks, E.K.; Ehrensberger, M.T. Bio-corrosion of magnesium alloys for orthopaedic applications. J. Funct. Biomater. 2017, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, M.; Li, Y.; Zhao, J.; Qin, L.; Lai, Y. Corrosion and biocompatibility improvement of magnesium-based alloys as bone implant materials: A review. Regen. Biomater. 2017, 4, 129–137. [Google Scholar] [CrossRef]
- Gonzalez, J.; Hou, R.Q.; Nidadavolu, E.P.; Willumeit-Romer, R.; Feyerabend, F. Magnesium degradation under physiological conditions—Best practice. Bioactive Mater. 2018, 3, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.Z.; Tjong, S.C. Rheology and morphology of compatibilized polyamide 6 blends containing liquid crystalline copolyesters. Polymer 1998, 39, 99–107. [Google Scholar] [CrossRef]
- Meng, Y.Z.; Tjong, S.C.; Hay, A.S.; Wang, S.J. Synthesis and proton conductivities of phosphonic acid containing poly-(arylene ether)s. J. Polym. Sci. A Polym. Chem. 2001, 39, 3218–3226. [Google Scholar] [CrossRef]
- Du, L.C.; Meng, Y.Z.; Wang, S.J.; Tjong, S.C. Synthesis and degradation behavior of poly(propylene carbonate) derived from carbon dioxide and propylene oxide. J. Appl. Polym. Sci. 2004, 92, 1840–1846. [Google Scholar] [CrossRef]
- Wei, Q.; Deng, N.N.; Guo, J.; Deng, J. Synthetic polymers for biomedical applications. Int. J. Biomater. 2018, 2018, 7158621. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 2018, 3117–3145. [Google Scholar] [CrossRef] [PubMed]
- Roeder, R.K.; Smith, S.M.; Conrad, T.L.; Yanchak, N.J.; Merrill, C.H.; Converse, G.L. Porous and bioactive PEEK implants for interbody spinal fusion. Adv. Mater. Process 2009, 67, 46–48. [Google Scholar]
- Shi, C.; Yuan, Z.; Han, F.; Zhu, C.; Li, B. Polymeric biomaterials for bone regeneration. Ann. Jt. 2016, 1, 27. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Silvipriya, K.S.; Krishna Kumar, K.; Bhat, A.R.; Dinesh Kumar, B.; John, A.; Lakshmanan, P. Collagen: Animal sources and biomedical application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Novitskaya, E.; Chen, P.Y.; Hamed, E.; Li, J.; Lubarda, V.A.; Jasiuk, I.; McKittrick, J. Recent advances on the measurement and calculation of the elastic moduli of cortical and trabecular bone: A review. Theoret. Appl. Mech. 2011, 38, 209–297. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Joseph, R.; Bonfield, W. Hydroxyapatite-polyethylene composites for bone substitution: Effects of ceramic particle size and morphology. Biomaterials 1998, 19, 2357–2366. [Google Scholar] [CrossRef]
- Russias, J.; Saiz, E.; Nalla, R.K.; Gryn, K.; Ritchie, R.O.; Tomsia, A.P. Fabrication and mechanical properties of PLA/HA composites: A study of in vitro degradation. Mater. Sci. Eng. C 2006, 26, 1289–1295. [Google Scholar] [CrossRef] [Green Version]
- Shikinami, Y.; Okuno, M. Bioresorbable devices made of forged composites of hydroxyapatite (HA) particles and poly-L-lactide (PLLA): Part I. Basic characteristics. Biomaterials 1999, 20, 859–877. [Google Scholar] [CrossRef]
- Wei, B.; Yao, Q.; Guo, Y.; Mao, F.; Liu, S.; Xu, Y.; Wang, L. Three-dimensional polycaprolactone–hydroxyapatite scaffolds combined with bone marrow cells for cartilage tissue engineering. J. Biomater. Appl. 2015, 30, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Tjong, S.C.; Meng, Y.Z. Morphology and mechanical characteristics of compatibilized polyamide 6-liquid crystalline polymer composites. Polymer 1997, 38, 4609–4615. [Google Scholar] [CrossRef]
- Li, R.K.Y.; Liang, J.Z.; Tjong, S.C. Morphology and dynamic mechanical properties of glass beads filled low density polyethylene composites. J. Mater. Process. Technol. 1998, 79, 59–65. [Google Scholar] [CrossRef]
- Tjong, S.C.; Liu, S.L.; Li, R.K.Y. Mechanical properties of injection moulded blends of polypropylene with thermotropic liquid crystalline polymer. J. Mater. Sci. 1996, 31, 479–484. [Google Scholar] [CrossRef]
- Tjong, S.C.; Chen, H. Nanocrystalline materials and coatings. Mater. Sci. Eng. R Rep. 2004, 45, 1–88. [Google Scholar] [CrossRef] [Green Version]
- He, L.X.; Tjong, S.C. Nanostructured transparent conductive films: Fabrication, characterization and applications. Mater. Sci. Eng. R Rep. 2016, 109, 1–101. [Google Scholar] [CrossRef]
- He, L.X.; Tjong, S.C. Aqueous graphene oxide-dispersed carbon nanotubes as inks for the scalable production of all-carbon transparent conductive films. J. Mater. Chem. C 2016, 4, 7043–7051. [Google Scholar] [CrossRef]
- Tjong, S.C. Nanocrystalline Materials: Their Synthesis-Structure-Property Relationships and Applications, 2nd ed.; Elsevier: London, UK, 2013; ISBN 9780124077966. [Google Scholar]
- Oliveira, A.E.; Braga, G.B.; Tarley, C.R.; Pereira, A.C. Thermally reduced graphene oxide: Synthesis, studies and characterization. J. Mater. Sci. 2018, 53, 12005–12015. [Google Scholar] [CrossRef]
- Li, A.; Xie, J.; Lie, J. Recent advances in functional nanostructured materials for bone-related diseases. J. Mater. Chem. B 2019, 7, 509–527. [Google Scholar] [CrossRef]
- Chieruzzi, M.; Pagano, S.; Moretti, S.; Pinna, R.; Milia, E.; Torre, L.; Eramo, S. Nanomaterials for tissue engineering in dentistry. Nanomaterials 2016, 6, 134. [Google Scholar] [CrossRef]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J. Biomed Mater. Res. 2000, 51, 475–483. [Google Scholar] [CrossRef]
- Hao, Y.; Yan, H.; Wang, X.; Zhu, B.; Ning, C.; Ge, S. Evaluation of osteoinduction and proliferation on nano-Sr-HAP: A novel orthopedic biomaterial for bone tissue regeneration. J. Nanosci. Nanotechnol. 2012, 12, 207–212. [Google Scholar] [CrossRef]
- Autefage, H.; Briand-Mesange, F.; Cazalbou, S.; Drouet, C.; Fourmy, D.; Goncalves, S.; Salles, J.P.; Combes, C.; Swider, P.; Rey, C. Adsorption and release of BMP-2 on nanocrystalline apatite-coated and uncoated hydroxyapatite/beta-tricalcium phosphate porous ceramics. J. Biomed. Mater. Res. Part B 2009, 91B, 706–715. [Google Scholar] [CrossRef]
- Liao, C.Z.; Li, K.; Wong, H.M.; Tong, W.Y.; Yeung, K.W.K.; Tjong, S.C. Novel polypropylene biocomposites reinforced with carbon nanotubes and hydroxyapatite nanorods for bone replacements. Mater. Sci. Eng. C 2013, 13, 1380–1388. [Google Scholar] [CrossRef]
- Liao, C.Z.; Wong, H.M.; Yeung, K.W.K.; Tjong, S.C. The development, fabrication and material characterization of polypropylene composites reinforced with carbon nanofiber and hydroxyapatite nanorod hybrid fillers. Int. J. Nanomed. 2014, 9, 1299–1310. [Google Scholar] [CrossRef]
- Chan, K.W.; Liao, C.Z.; Wong, H.M.; Yeung, K.W.K.; Tjong, S.C. Preparation of polyetheretherketone composites with nanohydroxyapatite rods and carbon nanofibers having high strength, good biocompatibility and excellent thermal stability. RSC Adv. 2016, 6, 19417–19429. [Google Scholar] [CrossRef]
- Liu, C.; Chan, K.W.; Shen, J.; Liao, C.Z.; Yeung, K.W.K.; Tjong, S.C. Polyetheretherketone hybrid composites with bioactive nanohydroxyapatite and multiwalled carbon nanotube fillers. Polymers 2016, 8, 425. [Google Scholar] [CrossRef]
- Liu, C.; Chan, K.W.; Shen, J.; Wong, H.M.; Yeung, K.W.; Tjong, S.C. Melt-compounded polylactic acid composite hybrids with hydroxyapatite nanorods and silver nanoparticles: Biodegradation, antibacterial ability, bioactivity and cytotoxicity. RSC Adv. 2015, 5, 72288–72299. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Ito, Y.; Zhang, P.; Chen, X. A comparative study on the in vivo degradation of poly(L-lactide) based composite implants for bone fracture fixation. Sci. Rep. 2016, 6, 20770. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Chang, W.; Lee, P.; Wang, Y.; Yang, M.; Li, J.; Kumbar, S.G.; Yu, X. Polymer-ceramic spiral structured scaffolds for bone tissue engineering: Effect of hydroxyapatite composition on human fetal osteoblasts. PLoS ONE 2014, 9, e85871. [Google Scholar] [CrossRef]
- Kutikov, A.B.; Gurijala, A.; Song, J. Rapid prototyping amphiphilic polymer/hydroxyapatite composite scaffolds with hydration-induced self-fixation behavior. Tissue Eng. Part C Methods 2015, 21, 229–241. [Google Scholar] [CrossRef]
- Lao, L.; Wang, Y.; Zhu, Y.; Zhang, Y.; Gao, C. Poly(lactide-co-glycolide)/hydroxyapatite nanofibrous scaffolds fabricated by electrospinning for bone tissue engineering. J. Mater. Sci. Mater. Med. 2011, 22, 1873–1884. [Google Scholar] [CrossRef]
- Wei, G.; Ma, P.X. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials 2004, 25, 4749–4757. [Google Scholar] [CrossRef]
- Kothapalli, C.R.; Shaw, M.T.; Wei, M. Biodegradable HA-PLA 3-D porous scaffolds: Effect of nano-sized filler content on scaffold properties. Acta Biomater. 2005, 1, 653–662. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.; Geim, A.K. Fines structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef]
- Chen, J.H.; Jang, C.; Xiao, S.; Ishigami, M.; Fuhrer, M.S. Intrinsic and extrinsic performance limits of graphene devices on SiO2. Nat. Nanotechnol. 2008, 3, 206–209. [Google Scholar] [CrossRef]
- Guazzo, R.; Gardin, C.; Bellin, G.; Sbricoli, L.; Ferroni, L.; Ludovichetti, F.S.; Piattelli, A.; Antoniac, I.; Bressan, E.; Zavan, B. Graphene-based nanomaterials for tissue engineering in the dental field. Nanomaterials 2018, 8, 349. [Google Scholar] [CrossRef]
- Liao, C.Z.; Li, Y.C.; Tjong, S.C. Graphene nanomaterials: Synthesis, biocompatibility, and cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Xu, H.; Xie, L.; Wu, D.; Hakkarainen, M. Immobilized graphene oxide nanosheets as thin but strong nanointerfaces in biocomposites. ACS Sustain. Chem. Eng. 2016, 4, 2211–2222. [Google Scholar] [CrossRef]
- Suk, J.W.; Piner, R.D.; An, J.; Ruoff, R.S. Mechanical properties of monolayer graphene oxide. ACS Nano 2010, 4, 6557–6564. [Google Scholar] [CrossRef]
- Ramesh, S.; Tan, C.Y.; Sopyan, I.; Hamdi, M.; Teng, W.D. Consolidation of nanocrystalline hydroxyapatite powder. Sci. Technol. Adv. Mater. 2007, 8, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Pinto, A.M.; Moreira, S.; Gonçalves, I.C.; Gama, F.M.; Mendes, A.M.; Magalhães, F.D. Biocompatibility of poly(lactic acid) with incorporated graphene-based materials. Colloids Surf. B Biointerfaces 2013, 104, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Adolfsson, K.H.; Xie, L.; Hassanzadeh, S.; Pettersson, T.; Hakkarainen, M. Zero-dimensional and highly oxygenated graphene oxide for multifunctional poly (lactic acid) bionanocomposites. ACS Sustain. Chem. Eng. 2016, 4, 5618–5631. [Google Scholar] [CrossRef]
- Bayer, I.S. Thermomechanical properties of polylactic acid-graphene composites: A state-of-the-art review for biomedical applications. Materials 2017, 10, 748. [Google Scholar] [CrossRef]
- Gonçalves, C.; Gonçalves, I.C.; Magalhães, F.D.; Pinto, A.M. Poly(lactic acid) composites containing carbon-based nanomaterials: A review. Polymers 2017, 9, 269. [Google Scholar] [CrossRef]
- An, X.; Ma, H.; Liu, B.; Wang, J. Graphene oxide reinforced polylactic acid/polyurethane antibacterial composites. J. Nanometer. 2013, 2013. Available online: https://www.hindawi.com/journals/jnm/2013/373414/ (accessed on 8 April 2019). [CrossRef]
- Wu, D.; Samanta, A.; Srivastava, R.K.; Hakkarainen, M. Nano-graphene oxide functionalized bioactive poly(lactic acid) and poly(ε-caprolactone) nanofibrous scaffolds. Materials 2018, 11, 566. [Google Scholar] [CrossRef]
- Yoon, O.J.; Jung, C.Y.; Sohn, I.Y.; Kim, H.J.; Hong, B.; Jhon, M.S.; Lee, N.-E. Nanocomposite nanofibers of poly(D, L-lactic-co-glycolic acid) and graphene oxide nanosheets. Compos. Part A 2011, 42, 1978–1984. [Google Scholar] [CrossRef]
- Luo, Y.; Shen, H.; Fang, Y.; Cao, Y.; Huang, J.; Zhang, M.; Dai, J.; Shi, X.; Zhang, Z. Enhanced proliferation and osteogenic differentiation of mesenchymal stem cells on graphene oxide-incorporated electrospun poly(lactic-co-glycolic acid) nanofibrous mats. ACS Appl. Mater. Interfaces 2015, 7, 6331–6339. [Google Scholar] [CrossRef]
- Erdal, N.B.; Hakkarainan, M. Construction of bioactive and reinforced bioresorbable nanocomposites by reduced nano-graphene oxide carbon dots. Biomacromolecules 2018, 19, 1074–1081. [Google Scholar] [CrossRef]
- Hassanzadeh, S.; Adolfsson, K.H.; Wu, D.; Hakkarainen, M. Supramolecular assembly of biobased graphene oxide quantum dots controls the morphology of and induces mineralization on poly(ε-caprolactone) films. Biomacromolecules 2016, 17, 256–261. [Google Scholar] [CrossRef]
- Liu, C.; Wong, H.M.; Yeung, K.W.; Tjong, S.C. Novel electrospun polylactic acid nanocomposite fiber mats with hybrid graphene oxide and nanohydroxyapatite reinforcements having enhanced biocompatibility. Polymers 2016, 8, 287. [Google Scholar] [CrossRef]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.L.; Ahn, J.H.; Hong, B.H.; Patorin, G. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef] [PubMed]
- Shadjou, N.; Hasanzadeth, M. Graphene and its nanostructure derivatives for use in bone tissue engineering: Recent advances. J. Biomed. Mater. Res. A 2016, 104, 1250–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, C.; Liu, Z.; Jiang, F.; Zeng, B.; Huang, M.; Yu, D. Cellular behaviours of bone marrow-derived mesenchymal stem cells towards pristine graphene oxide nanosheets. Cell Prolif. 2017, 50, e12367. [Google Scholar] [CrossRef]
- Halim, A.; Luo, Q.; Ju, Y.; Song, G. A Mini review focused on the recent applications of graphene oxide in stem cell growth and differentiation. Nanomaterials 2018, 8, 736. [Google Scholar] [CrossRef] [PubMed]
- Tjong, S.C. Advances in Biomedical Science and Engineering; Bentham: New York, NY, USA, 2009. [Google Scholar]
- Li, K.; Tjong, S.C. Hydrothermal synthesis and biocompatibility of hydroxyapatite nanorods. J. Nanosci. Nanotechnol. 2011, 11, 10444–110448. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Natasha, A.N.; Tan, C.Y.; Bang, L.T.; Niakan, A.; Purbolaksono, J.; Chandran, H.; Ching, C.Y.; Ramesh, S.; Teng, W.D. Characteristics and properties of hydoxyapatite derived by sol–gel and wet chemical precipitation methods. Ceram. Int. 2015, 41, 10434–10441. [Google Scholar] [CrossRef]
- Rodrıguez-Lugo, V.; Karthik, T.V.; Mendoza-Anaya, D.; Rubio-Rosas, E.; Ceron, L.S.; Reyes-Valderrama, M.I.; Salinas-Rodrıguez, E. Wet chemical synthesis of nanocrystalline hydroxyapatite flakes: Effect of pH and sintering temperature on structural and morphological properties. R. Soc. Open Sci. 2018, 5, 180962. [Google Scholar] [CrossRef]
- Nagata, F.; Yamauchi, Y.; Tomita, M.; Kato, K. Hydrothermal synthesis of hydroxyapatite nanoparticles and their protein adsorption behavior. J. Ceram. Soc. Jpn. 2013, 121, 797–801. [Google Scholar] [CrossRef] [Green Version]
- Xue, C.; Chen, Y.; Huang, Y.; Zhu, P. Hydrothermal synthesis and biocompatibility study of highly crystalline carbonated hydroxyapatite nanorods. Nanoscale Res. Lett. 2015, 10, 316. [Google Scholar] [CrossRef]
- Siddharthan, A.; Seshadri, S.K.; Sampath Kumar, T.S. Influence of microwave power on nanosized hydroxyapatite particles. Scrip. Mater. 2006, 55, 175–178. [Google Scholar] [CrossRef]
- Kalita, S.J.; Verma, S. Nanocrystalline hydroxyapatite bioceramic using microwave radiation: Synthesis and characterization. Mater. Sci. Eng. C 2010, 30, 295–303. [Google Scholar] [CrossRef]
- Hassan, M.; Mahmood, M.M.; El-Fattah, A.A.; Kandil, S. Microwave-assisted preparation of nano-hydroxyapatite for bone substitutes. Ceram. Int. 2016, 42, 3725–3744. [Google Scholar] [CrossRef]
- Skakalova, V.; Kotrusz, P.; Jergel, M.; Susi, T.; Mittelberger, A.; Vretenar, V.; Siffalovic, P.; Kotakoski, J.; Meyer, J.C.; Hulman, M. Chemical oxidation of graphite: Evolution of the structure and properties. J. Phys. Chem. C 2018, 122, 929–935. [Google Scholar] [CrossRef]
- Feicht, P.; Eigler, S. Defects in graphene oxide as structural motif. ChemNanoMat 2018, 4, 244–252. [Google Scholar] [CrossRef]
- Guerrero-Contreras, J.; Caballero-Briones, F. Graphene oxide powders with different oxidation degree, prepared by synthesis variations of the Hummers method. Mater. Chem. Phys. 2015, 153, 209–220. [Google Scholar] [CrossRef]
- Wong, C.A.; Sofer, Z.; Kubesovac, M.; Kucera, J.; Matejkova, S.; Pumera, M. Synthetic routes contaminate graphene materials with a whole spectrum of unanticipated metallic elements. Proc. Nat. Acad. Sci. USA 2014, 111, 13774–13779. [Google Scholar] [CrossRef] [Green Version]
- Gunter, T.E.; Gavin, C.E.; Gunter, K.K. The case for manganese interaction with mitochondria. Neurotoxicology 2009, 30, 727–729. [Google Scholar] [CrossRef]
- Muzyka, R.; Drewniak, S.; Pustelny, T.; Chrubasik, M.; Gryglewicz, G. Characterization of graphite oxide and reduced graphene oxide obtained from different graphite precursors and oxidized by different methods using Raman spectroscopy. Materials 2018, 11, 1050. [Google Scholar] [CrossRef]
- Peng, L.; Xu, Z.; Liu, Z.; Wei, Y.; Sun, H.; Li, Z.; Zha, X.; Gao, C. An iron-based green approach to 1-h production of single-layer graphene oxide. Nat. Commun. 2015, 6, 5716. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.T.; Zhang, B.; Li, R.; Xing, R. High-efficient synthesis of graphene oxide based on improved Hummers method. Sci. Rep. 2016, 6, 36143. [Google Scholar] [CrossRef]
- Yoon, H.W.; Cho, Y.H.; Park, H.B. Graphene-based membranes: status and prospects. Philos. Trans. R. Soc. A 2016, 374, 20150024. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.Y.; Forster, M.; Klinowski, J. Structure of graphite oxide revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascon, J.M. Graphene oxide dispersions in organic solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-reduction of graphite- and graphene oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- McAllister, M.J.; Li, J.L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K.; et al. Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Mattevi, C.; Eda, G.; Agnoli, S.; Miller, S.; Mkhoyan, K.A.; Celik, O.; Mastrogiovanni, D.; Granozzi, G.; Garfunkel, E.; Chhowalla, M. Evolution of Electrical, Chemical, and Structural Properties of Transparent and Conducting Chemically Derived Graphene Thin Films. Adv. Func. Mater. 2009, 19, 2577–2583. [Google Scholar] [CrossRef]
- Tang, D.; Liu, J.; Yang, X.; Kang, L. Graphene oxide derived graphene quantum dots with different photoluminescence properties and peroxidase-like catalytic activity. RSC Adv. 2017, 6, 50609–50617. [Google Scholar] [CrossRef]
- Ahirwar, S.; Mallick, S.; Bahadur, D. Electrochemical method to prepare graphene quantum dots and graphene oxide quantum dots. ACS Omega 2017, 2, 8343–8353. [Google Scholar] [CrossRef]
- Tabish, T.A.; Scotton, C.J.; Ferguson, D.C.; Lin, L.; van der Veen, A.; Lowry, S.; Ali, M.; Jabeen, F.; Ali, M.; Winyard, P.G.; et al. Biocompatibility and toxicity of graphene quantum dots for potential application in photodynamic therapy. Nanomedicine 2018, 13, 1923–1937. [Google Scholar] [CrossRef]
- Roy, P.; Periasamy, A.P.; Lin, C.Y.; Her, G.M.; Chiu, W.J.; Li, C.L.; Shu, C.L.; Huang, C.C.; Liang, C.T.; Chang, H.T. Photoluminescent graphene quantum dots for in vivo imaging of apoptotic cells. Nanoscale 2015, 7, 2504–2510. [Google Scholar] [CrossRef]
- Pan, D.Y.; Zhang, J.C.; Li, Z.; Wu, M.H. Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef]
- Zhao, M. Direct Synthesis of graphene quantum dots with different fluorescence properties by oxidation of graphene oxide using nitric acid. Appl. Sci. 2018, 8, 1303. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, X.; Sun, S.; Ma, L.; Zhang, L.; Lin, H. A facile and high-efficient approach to yellow emissive graphene quantum dots from graphene oxide. Carbon 2017, 124, 342–347. [Google Scholar] [CrossRef]
- Li, L.L.; Ji, J.; Fei, R.; Wang, C.Z.; Lu, Q.; Zhang, J.R.; Jiang, L.P.; Zhu, J.J. A Facile microwave avenue to electrochemiluminescent two-color graphene quantum dots. Adv. Funct. Mater. 2012, 22, 2971–2979. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Qiao, C.; Tang, S.; Li, Y.; Yuan, W.; Li, B.; Tian, L.; Liu, F.; Hu, R.; et al. Strongly green-photoluminescent graphene quantum dots for bioimaging applications. Chem. Commun. 2011, 47, 6858–6860. [Google Scholar] [CrossRef]
- Ma, H.; Su, W.; Tai, Z.; Sun, D.; Yan, X.; Liu, B.; Xu, Q. Preparation and cytocompatibility of polylactic acid/hydroxyapatite/graphene oxide nanocomposite fibrous membrane. Chin. Sci. Bull. 2012, 57, 3051–3058. [Google Scholar] [CrossRef] [Green Version]
- Marques, P.A.; Gonçalves, G.; Singh, M.K.; Gracio, J. Graphene oxide and hydroxyapatite as fillers of polylactic acid nanocomposites: Preparation and characterization. J. Nanosci. Nanotechnol. 2012, 12, 6686–6692. [Google Scholar] [CrossRef]
- Liang, C.; Luo, Y.; Yang, G.; Xia, D.; Liu, L.; Zhang, X.; Wang, H. Graphene oxide hybridized nHAC/PLGA scaffolds facilitate the proliferation of MC3T3-E1 cells. Nanoscale Res. Lett. 2018, 13, 15. [Google Scholar] [CrossRef]
- Murugan, N.; Murugan, C.; Sundramoorthy, A.K. In vitro and in vivo characterization of mineralized hydroxyapatite/polycaprolactone-graphene oxide based bioactive multifunctional coating on Ti alloy for bone implant applications. Arab J. Chem. 2017, 11, 959–969. [Google Scholar] [CrossRef]
- Gong, M.; Zhao, Q.; Dai, L.; Li, Y.; Jiang, T. Fabrication of polylactic acid/hydroxyapatite/graphene oxide composite and their thermal stability, hydrophobic and mechanical properties. J. Asian Ceram. Soc. 2017, 5, 160–168. [Google Scholar] [CrossRef]
- Fu, C.; Bai, H.; Zhu, J.; Niu, Z.; Wang, Y.; Li, J.; Yang, X.; Bai, Y. Enhanced cell proliferation and osteogenic differentiation in electrospun PLGA/hydroxyapatite nanofibre scaffolds incorporated with graphene oxide. PLoS ONE 2017, 12, e0188352. [Google Scholar] [CrossRef]
- Mohandes, F.; Salavati-Niasari, M. In vitro comparative study of pure hydroxyapatite nanorods and novel polyethylene glycol/graphene oxide/hydroxyapatite nanocomposite. J. Nanopart. Res. 2014, 16, 2604. [Google Scholar] [CrossRef]
- Rodríguez-Gonzalez, C.; Cid-Luna, H.E.; Salas, P.; Castano, V.M. Hydroxyapatite functionalized graphene: A new hybrid nanomaterial. J. Nanomater. 2014, 2014, 940903. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, J.; Wang, Z.; Ran, H.; Li, Y.; Niu, L.; Gong, P.; Liu, B.; Yang, S. One-pot synthesis of graphene/hydroxyapatite nanorod composite for tissue engineering. Carbon 2014, 66, 407–416. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Liu, Q.; Li, Q.; Cheng, Y.; Zheng, Y.; Xi, T.; Wei, S. In situ synthesis and biocompatibility of nanohydroxyapatite on pristine and chitosan functionalized graphene oxide. J. Mater. Chem. B 2013, 1, 475–484. [Google Scholar] [CrossRef]
- Li, M.; Xiong, P.; Yan, F.; Li, S.; Ren, C.; Yin, Z.; Li, A.; Li, H.; Ji, X.; Zheng, Y.; et al. An overview of graphene-based hydroxyapatite composites for orthopedic applications. Bioactive Mater. 2018, 3, 1–18. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, Y.C.; Lee, S.M.; Jin, O.S.; Kang, S.H.; Hong, S.W.; Jeong, C.M.; Huh, J.B.; Han, D.W. Enhanced osteogenesis by reduced graphene oxide/hydroxyapatite nanocomposites. Sci. Rep. 2015, 5, 18833. [Google Scholar] [CrossRef]
- Nie, W.; Peng, C.; Zhou, X.; Chen, L.; Wang, W.; Zhang, Y.; Ma, P.X.; He, C. Three-dimensional porous scaffold by self-assembly of reduced graphene oxide and nano-hydroxyapatite composites for bone tissue engineering. Carbon 2017, 116, 325–337. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Tran, R.T.; Naseri, E.; Kolasnikov, A.; Bai, X.; Yang, J. A new generation of sodium chloride porogen for tissue engineering. Biotechnol. Appl. Biochem. 2011, 58, 335–344. [Google Scholar] [CrossRef]
- Poursamar, S.A.; Hatami, J.; Lehner, A.N.; da Silva, C.L.; Ferreira, F.C.; Antunes, A.P. Gelatin porous scaffolds fabricated using a modified gas foaming technique: Characterisation and cytotoxicity assessment. Mater. Sci. Eng. C 2015, 48, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Liao, X.; Zhang, H.; He, T. Preparation of porous biodegradable polymer and its nanocomposites by supercritical CO2 foaming for tissue engineering. J. Nanometer. 2012, 2012, 836394. [Google Scholar] [CrossRef]
- Haugh, M.G.; Murphy, C.M.; O’Brien, F.J. Novel freeze-drying methods to produce a range of collagen-glycosaminoglycan scaffolds with tailored mean pore sizes. Tissue Eng. Part C Methods 2010, 16, 887–894. [Google Scholar] [CrossRef]
- Conoscenti, G.; Carrubba, V.; Brucato, V. A versatile technique to produce porous polymeric scaffolds: The thermally induced phase separation (TIPS) method. Arch. Chem. Res. 2017, 1, 2. [Google Scholar] [CrossRef]
- Gay, S.; Lefebvre, G.; Bonnin, M.; Nottelet, B.; Boury, F.; Gibaud, A.; Calvignac, B. PLA scaffolds production from thermally induced phase separation: Effect of process parameters and development of an environmentally improved route assisted by supercritical carbon dioxide. J. Supercrit. Fluid 2018, 136, 123–135. [Google Scholar] [CrossRef]
- Tamayol, A.; Akbari, M.; Annabi, N.; Khademhosseini, A.; Junker, D. Fiber-based tissue engineering: Progress, challenges, and opportunities. Biotechnol. Adv. 2013, 31, 669–687. [Google Scholar] [CrossRef] [Green Version]
- Malikmammadov, E.; Tanir, T.A.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL-TCP wet spun scaffolds carrying antibiotic-loaded microspheres for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2018, 29, 805–824. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.S.; Edwards, J.R.; Jeon, H. Electrospun fibrous scaffolds for tissue engineering: Viewpoints on architecture and fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef]
- Rajzer, I.; Menaszek, E.; Castano, O. Electrospun polymer scaffolds modified with drugs for tissue engineering. Mater. Sci. Eng. C 2017, 77, 493–499. [Google Scholar] [CrossRef]
- Peng, F.; Yu, X.; Wei, M. In vitro cell performance on hydroxyapatite particles/poly(L-lactic acid) nanofibrous scaffolds with an excellent particle along nanofiber orientation. Acta Biomater. 2011, 7, 2585–2592. [Google Scholar] [CrossRef]
- Keivani, F.; Shokrollahi, P.; Zandi, M.; Irani, S.; Shokrolahi, F.; Khorasani, S.C. Engineered electrospun poly(caprolactone)/polycaprolactone-g-hydroxyapatite nano-fibrous scaffold promotes human fibroblasts adhesion and proliferation. Mater. Sci. Eng. C 2016, 68, 78–88. [Google Scholar] [CrossRef]
- Parmaksiz, M.; Elcina, A.E.; Elcina, Y.M. Decellularized bovine small intestinal submucosa-PCL/hydroxyapatite based multilayer composite scaffold for hard tissue repair. Mater. Sci. Eng. C 2019, 94, 788–797. [Google Scholar] [CrossRef]
- Kwon, I.K.; Kidoaki, S.; Matsuda, T. Electrospun nano- to microfiber fabrics made of biodegradable copolyesters: Structural characteristics, mechanical properties and cell adhesion potential. Biomaterials 2005, 26, 3929–3939. [Google Scholar] [CrossRef]
- Marenzana, M.; Arnett, T.R. The key role of the blood supply to bone. Bone Res. 2013, 3, 203–215. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef]
- He, Y.; Tuck, C.J.; Prina, E.; Kilsby, S.; Christie, S.D.; Edmondson, S.; Hague, R.J.; Rose, F.R.; Wildman, R.R. A new photocrosslinkable polycaprolactone-based ink for three-dimensional inkjet printing. J. Biomed Mater. Res. B 2017, 105, 1645–1657. [Google Scholar] [CrossRef]
- Moncal, K.K.; Heo, D.N.; Godzik, K.P.; Sosnoski, D.M.; Mrowczynski, O.D.; Rizk, E.; Ozbolat, V.; Tucker, S.M.; Gerhard, E.M.; Dey, M.; et al. 3D printing of poly(ε caprolactone)/poly(D,L-lactide-co-glycolide)/hydroxyapatite composite constructs for bone tissue engineering. J. Mater. Res. 2018, 33, 1972–1986. [Google Scholar] [CrossRef]
- Du, Y.; Liu, H.; Yang, Q.; Wang, S.; Wang, J.; Ma, J.; Noh, I.; Mikos, A.G.; Zhang, S. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials 2017, 137, 37–48. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Liao, C.; Tjong, S.C. Scalable fabrication of high-performance transparent conductors using graphene oxide-stabilized single-walled carbon nanotube inks. Nanomaterials 2018, 8, 224. [Google Scholar] [CrossRef]
- Jakus, A.E.; Secor, E.B.; Rutz, A.L.; Jordan, S.W.; Hersam, M.C.; Shah, R.N. Three-dimensional printing of high-content graphene scaffolds for electronic and biomedical applications. ACS Nano 2015, 9, 4636–4648. [Google Scholar] [CrossRef]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.C.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Diseases 2017, 4, 185–195. [Google Scholar] [CrossRef]
- Datta, P.; Ayan, B.; Ozbolat, I.T. Bioprinting for vascular and vascularized tissue biofabrication. Acta Biomater. 2017, 51, 1–20. [Google Scholar] [CrossRef]
- Zhu, W.; Qu, X.; Zhu, J.; Ma, X.; Patel, S.; Liu, J.; Wang, P.; Lai, C.S.; Gou, M.; Xu, Y.; et al. Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials 2018, 124, 106–115. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Feng, L.; Wu, J.; Tan, Z.; Yao, R. Bioprinting of stem cells: Interplay of bioprinting process, bioinks, and stem cell properties. ACS Biomater. Sci. Eng. 2018, 4, 3108–3124. [Google Scholar] [CrossRef]
- Gao, B.; Yang, Q.; Zhao, X.; Jin, G.; Ma, Y.; Xu, F. 4D bioprinting for biomedical applications. Trends Biotechnol. 2016, 34, 746–756. [Google Scholar] [CrossRef]
- Li, X.; Shang, J.; Wang, Z. Intelligent materials: A review of applications in 4D printing. Assem. Autom. 2017, 37, 170–185. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical applications of biodegradable polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef]
- Okamoto, M.; John, B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Sun, X.; Xu, C.; Wu, G.; Ye, Q.; Wang, C. Poly(lactic-co-glycolic acid): Applications and future prospects for periodontal tissue regeneration. Polymers 2017, 9, 189. [Google Scholar] [CrossRef]
- Pilloni, A.; Pompa, G.; Saccucci, M.; Di Carlo, G.; Rimondini, L.; Brama, M.; Zeza, B.; Wannenes, F.; Migliaccio, S. Analysis of human alveolar osteoblast behavior on a nano-hydroxyapatite substrate: An in vitro study. BMC Oral Health 2014, 14, 22. [Google Scholar] [CrossRef]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef]
- Shi, Z.; Huang, X.; Cai, Y.; Tang, R.; Yang, D. Size effect of hydroxyapatite nanoparticles on proliferation and apoptosis of osteoblast-like cells. Acta Biomater. 2009, 5, 338–345. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, Y.; Yan, W.; Hu, Q.; Tao, J.; Zhang, M.; Shi, Z.; Tang, R. Role of hydroxyapatite nanoparticle size in bone cell proliferation. J. Mater. Chem. 2007, 17, 3780–3787. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Liu, X.; Zhang, R.; Feng, Q. In vitro Uptake of hydroxyapatite nanoparticles and their effect on osteogenic differentiation of human mesenchymal stem cells. Stem Cells Int. 2018, 2018, 2036176. [Google Scholar] [CrossRef]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, J.; Lowe, B.; Anil, S.; Kim, S.K.; Shim, M.S. Combination of nano-hydroxyapatite with stem cells for bone tissue engineering. J. Nanosci. Nanotechnol. 2016, 16, 8881–8894. [Google Scholar] [CrossRef]
- Michel, J.; Penna, M.; Kochen, J.; Cheung, H. Recent advances in hydroxyapatite scaffolds containing mesenchymal stem cells. Stem Cells Int. 2015, 2015, 305217. [Google Scholar] [CrossRef]
- Dubey, N.; Bentini, R.; Islam, I.; Cao, T.; Neto, A.H.; Rosa, V. Graphene: A versatile carbon-based material for bone tissue engineering. Stem Cells Int. 2015, 2015, 804213. [Google Scholar] [CrossRef]
- Garcia-Alegria, E.; Iliut, M.; Stefanska, M.; Silva, C.; Heeg, S.; Kimber, S.J.; Kouskoff, V.; Lacaud, G.; Vijayaraghavan, A.; Batta, K. Graphene oxide promotes embryonic stem cell differentiation to haematopoietic lineage. Sci. Rep. 2016, 6, 25917. [Google Scholar] [CrossRef]
- Verre, A.F.; Faroni, A.; Iliut, M.; Silva, C.; Muryn, C.; Reid, A.J.; Vijayaraghavan, A. Improving the glial differentiation of human Schwann-like adipose-derived stem cells with graphene oxide substrates. Interface Focus 2018, 8, 20180002. [Google Scholar] [CrossRef] [Green Version]
- Michael, F.M.; Ratnam, C.T.; Khalid, M.; Ramarad, S.; Walvekar, R. Surface modification of nanohydroxyapatite and its loading effect on polylactic acid properties for load bearing implants. Polym. Compos. 2017, 39, 2880–2888. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Bone tissue regeneration: The role of scaffold geometry. Biomater. Sci. 2015, 3, 231–245. [Google Scholar] [CrossRef]
- Nejati, E.; Mirzadeh, H.; Zandi, M. Synthesis and characterization of nano-hydroxyapatite rods/poly(L-lactide acid) composite scaffolds for bone tissue engineering. Compos. Part A 2008, 39, 1589–1596. [Google Scholar] [CrossRef]
- Haj, J.; Khalil, T.H.; Falah, M.; Zussman, E.; Srouji, S. An ECM-mimicking, mesenchymal stem cell-embedded hybrid scaffold for bone regeneration. BioMed. Res. Int. 2017, 2017, 8591073. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, L.; Cheng, R.; Cui, W. ECM decorated electrospun nanofiber for improving bone tissue regeneration. Polymers 2018, 10, 272. [Google Scholar] [CrossRef]
- Jeong, S.I.; Ko, E.K.; Yum, J.; Jung, C.H.; Lee, Y.M.; Shin, H. Nanofibrous poly(lactic acid)/hydroxyapatite composite scaffolds for guided tissue regeneration. Macromol. Biosci. 2008, 8, 328–338. [Google Scholar] [CrossRef]
- Marrella, A.; Lee, T.Y.; Lee, D.H.; Karuthedom, S.; Syla, D.; Chawla, A.; Khademhosseini, A.; Jang, H.L. Engineering vascularized and innervated bone biomaterials for improved skeletal tisuue regeneration. Mater. Today 2018, 21, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Morelli, S.; Salerno, S.; Holopainen, J.; Ritala, M.; De Bartolo, L. Osteogenic and osteoclastogenic differentiation of co-cultured cells in polylactic acid–nanohydroxyapatite fiber scaffolds. J. Biotechnol. 2015, 204, 53–62. [Google Scholar] [CrossRef]
- An, J.; Teoh, J.E.; Suntornnond, R.; Chua, C.K. Design and 3D printing of scaffolds and tissues. Engineering 2015, 1, 261–268. [Google Scholar] [CrossRef]
- Wenger, R.; Giraud, M.N. 3D printing applied to tissue engineered vascular grafts. Appl. Sci. 2018, 8, 2631. [Google Scholar] [CrossRef]
- Gremare, A.; Guduric, V.; Bareille, R.; Heroguez, V.; Latour, S.; L’heureux, N.; Fricain, J.C.; Catros, S.; Le Nihouannen, D. Characterization of printed PLA scaffolds for bone tissue engineering. J. Biomed. Mater. Res. A 2018, 106, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Gregor, A.; Filova, E.; Novak, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnova, V.; Lukasova, V.; Bartos, M.; Necas, A.; et al. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Mao, X.; Du, Z.; Jiang, W.; Han, X.; Zhao, D.; Han, D.; Li, Q. Three dimensional printed macroporous polylactic acid/hydroxyapatite composite scaffolds for promoting bone formation in a critical-size rat calvarial defect model. Sci. Technol. Adv. Mater. 2016, 17, 136–148. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Xiong, J.; Liu, J.; Zhua, W.; Chen, J.; Duan, L.; Zhang, J.; Wang, D. Evaluation of the novel three-dimensional porous poly (L-lactic acid)/nanohydroxyapatite composite scaffold. Biomed. Mater. Eng. 2015, 26, S197–S205. [Google Scholar] [CrossRef]
- Corcione, E.C.; Gervaso, F.; Scalera, F.; Montagna, F.; Sannino, A.; Maffezzoli, A. The feasibility of printing polylactic acid–nanohydroxyapatite composites using a low-cost fused deposition modeling 3D printer. J. Appl. Polym. Sci. 2017, 134, 44656. [Google Scholar] [CrossRef]
- Rodrigues, N.; Benning, M.; Ferreira, A.M.; Dixon, L.; Dalgarno, K. Manufacture and Characterisation of Porous PLA Scaffolds. Procedia CIRP 2016, 49, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Pinto, A.M.; Cabral, J.; Tanaka, D.P.; Mendes, A.M.; Magalhaes, F.D. Effect of graphene oxide and graphene nanoplatelets incorporation on mechanical and gas permeability properties of poly(lactic acid) films. Polym. Int. 2013, 62, 33–40. [Google Scholar] [CrossRef]
- Arriagada, P.; Palza, H.; Palma, P.; Flores, M.; Caviedes, P. Poly(lactic acid) composites based on graphene oxide particles with antibacterial behavior enhanced by electrical stimulus and biocompatibility. J. Biomed. Mater. Res. A 2018, 106, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Chartarrayawade, W.; Molloy, R.; Ratchawet, A.; Janmee, N.; Butsamran, M.; Panpai, K. Fabrication of poly(lactic acid)/graphene oxide/stearic acid composites with improved tensile strength. Polym. Compos. 2017, 38, 2272–2282. [Google Scholar] [CrossRef]
- Li, W.; Xu, J.; Chen, L.; Shan, M.; Tian, X.; Yang, C.; Lv, H.; Qian, X. A facile method to produce graphene oxide-g-poly(L-lactic acid) as an promising reinforcement for PLLA nanocomposites. Chem. Eng. J. 2014, 237, 291–299. [Google Scholar] [CrossRef]
- Deng, X.; Zeng, Z.; Peng, B.; Yan, S.; Ke, W. Mechanical properties optimization of poly-ether-ether-ketone via fused deposition modeling. Materials 2018, 11, 216. [Google Scholar] [CrossRef]
- Chen, Q.; Mangadlao, J.D.; Wallat, J.; De Leon, A.; Pokorski, J.K.; Advincula, R.C. 3D printing biocompatible polyurethane/poly(lactic acid)/graphene oxide nanocomposites: Anisotropic properties. ACS Appl. Mater. Interfaces 2017, 9, 4015–4023. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Li, J.; Huang, W.; Jiang, H.; Zimba, B.L.; Chen, L.; Wan, J.; Wu, Q. Preparation of poly(lactic acid)/graphene oxide nanofiber membranes with different structures by electrospinning for drug delivery. RSC Adv. 2018, 8, 16619–16625. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, L.; Zhai, T.; Wang, X.; Dan, Y.; Turng, L.S. The surface grafting of graphene oxide with poly (ethylene glycol) as a reinforcement for poly (lactic acid) nanocomposite scaffolds for potential tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2016, 53, 403–413. [Google Scholar] [CrossRef]
- Chen, C.; Sun, X.; Pan, W.; Hou, Y.; Liu, R.; Jiang, X.; Zhang, L. Graphene oxide-templated synthesis of hydroxyapatite nanowhiskers to improve the mechanical and osteoblastic performance of poly(lactic acid) for bone tissue regeneration. ACS Sustain. Chem. Eng. 2018, 6, 3862–3869. [Google Scholar] [CrossRef]
- Hernandez, C.J. Cancellous Bone. In Handbook of Biomaterial Properties; Murphy, W., Black, J., Hastings, G., Eds.; Springer Science: New York, NY, USA, 2016; pp. 15–21. [Google Scholar]
- Yu, P.; Bao, R.Y.; Shi, X.J.; Yang, W.; Yang, M.B. Self-assembled high-strength hydroxyapatite/graphene oxide/chitosan composite hydrogel for bone tissue engineering. Carbohydr. Polym. 2017, 155, 507–515. [Google Scholar] [CrossRef]
- Uddin, M.J.; Middya, T.R.; Chaudhuri, B.K. Preparation of silver-hydroyapatite/PVA nanocomposites: Giant dielectric material for industrial and clinical applications. IOP Conf. Ser. Mater. Sci. Eng 2015, 73, 012070. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Shen, J.; Liao, C.Z.; Yeung, K.W.K.; Tjong, S.C. Novel electrospun polyvinylidene fluoride-graphene oxide-silver nanocomposite membranes with protein and bacterial antifouling characteristics. Express Polym. Lett. 2018, 12, 365–382. [Google Scholar] [CrossRef]
- Kim, S.S.; Park, M.S.; Jeon, O.; Choi, C.Y.; Kim, B.S. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 1399–1409. [Google Scholar] [CrossRef]
- Jose, M.V.; Thomas, V.; Johnson, K.T.; Dean, D.R.; Nyairo, E. Aligned PLGA/HA nanofibrous nanocomposite scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 305–315. [Google Scholar] [CrossRef]
- Li, M.; Liu, W.; Sun, J.; Xianyu, Y.; Wang, J.; Zhang, W.; Zheng, W.; Huang, D.; Di, S.; Long, Y.Z.; et al. Culturing primary human osteoblasts on electrospun poly(lactic-coglycolic acid) and poly(lactic-co-glycolic acid)/nanohydroxyapatite scaffolds for bone tissue engineering. ACS Appl. Mater. Interfaces 2013, 5, 5921–5926. [Google Scholar] [CrossRef]
- De Mori, A.; Peña Fernández, M.; Blunn, G.; Tozzi, G.; Roldo, M. 3D printing and electrospinning of composite hydrogels for cartilage and bone tissue engineering. Polymers 2018, 10, 285. [Google Scholar] [CrossRef]
- Moeini, S.; Mohammadi, M.R.; Simchi, A. In-situ solvothermal processing of polycaprolactone/hydroxyapatite nanocomposites with enhanced mechanical and biological performance for bone tissue engineering. Bioact. Mater. 2017, 2, 146–155. [Google Scholar] [CrossRef]
- Kumar, S.; Bose, S.; Chatterjee, K. Amine-functionalized multiwall carbon nanotubes impart osteoinductive and bactericidal properties in poly(caprolactone) composites. RSC Adv. 2014, 4, 19086. [Google Scholar] [CrossRef]
- Eshraghi, S.; Das, S. Mechanical and microstructural properties of polycaprolactone scaffolds with one-dimensional, two-dimensional, and three-dimensional orthogonally oriented porous architectures produced by selective laser sintering. Acta Biomater. 2010, 6, 2467–2476. [Google Scholar] [CrossRef]
- Li, H.; Huang, C.; Jin, X.; Ke, Q. An electrospun poly(ε-caprolactone) nanocomposite fibrous mat with a high content of hydroxyapatite to promote cell infiltration. RSC Adv. 2018, 8, 25228. [Google Scholar] [CrossRef]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.J.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef]
- Xia, Y.; Zhou, P.; Cheng, X.; Xie, Y.; Liang, C.; Li, C.; Xu, S. Selective laser sintering fabrication of nano-hydroxyapatite/poly-ε-caprolactone scaffolds for bone tissue engineering applications. Int. J. Nanomed. 2013, 8, 4197–4213. [Google Scholar] [CrossRef] [Green Version]
- Heo, S.J.; Kim, S.J.; Wei, J.; Hyun, Y.T.; Yun, H.S.; Kim, D.H.; Shin, J.W. Fabrication and characterization of novel nano- and micro-HA/PCL composite scaffolds using a modified rapid prototyping process. J. Biomed. Mater. Res. 2009, 89A, 108–116. [Google Scholar] [CrossRef]
- Huang, B.; Caetano, G.; Vyas, C.; Blaker, J.J.; Diver, C.; Bártolo, P. Polymer-ceramic composite scaffolds: The effect of hydroxyapatite and β-tricalcium phosphate. Materials 2018, 11, 129. [Google Scholar] [CrossRef]
- Mota, C.; Gazzarri, M.; Dinucci, D.; Gloria, A.; Myrzabekova, M.; Ambrosio, L.; Chiellini, F. Additive manufacturing of wet-spun polymeric scaffolds for bone tissue engineering. Biomed. Microdevices 2012, 14, 1115–1127. [Google Scholar] [CrossRef]
- Puppi, D.; Migone, C.M.; Grassi, L.; Pirosa, A.; Maisetta, G.; Batoni, G.; Chiellini, F. Integrated 3D fibers/hydrogel biphasic scaffolds for periodontal bone tissue engineering. Polym. Int. 2016, 65, 631–640. [Google Scholar] [CrossRef]
- Dini, F.; Barsotti, G.; Puppi, D.; Coli, A.; Briganti, A.; Giannessi, E.; Miragliotta, V.; Mota, C.; Pirosa, A.; Stornelli, M.; et al. Tailored star poly (ε-caprolactone) wet-spun scaffolds for in vivo regeneration of long bone critical size defects. J. Bioact. Comp. Polym. 2016, 31, 15–30. [Google Scholar] [CrossRef]
- Kim, J.W.; Shin, J.H.; Koh, Y.H.; Hah, M.J.; Moon, J.; Kim, H.E. Production of poly(ε-caprolactone)/hydroxyapatite composite scaffolds with a tailored macro/micro-porous structure, high mechanical properties, and excellent bioactivity. Materials 2017, 10, 1123. [Google Scholar] [CrossRef]
- Pei, X.; Ma, L.; Zhang, B.; Sun, J.; Sun, Y.; Fan, Y.; Gou, Z.; Zhou, C.; Zhang, X. Creating hierarchical porosity hydroxyapatite scaffolds with osteoinduction by three-dimensional printing and microwave sintering. Biofabrication 2017, 9, 045008. [Google Scholar] [CrossRef]
- Lan Levengood, S.K.; Polak, S.J.; Wheeler, M.B.; Maki, A.J.; Clark, S.G.; Jamison, R.D.; Wagoner Johnson, A.J. Multiscale osteointegration as a new paradigm for the design of calcium phosphate scaffolds for bone regeneration. Biomaterials 2010, 31, 3552–3563. [Google Scholar] [CrossRef]
- Ma, H.; Cheng, C.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018, 79, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Domingos, M.; Intranuovo, F.; Russo, T.; De Santis, R.; Gloria, A.; Ambrosio, L.; Siurana, J.; Bartolo, P. The first systematic analysis of 3D rapid prototyped poly(ε-caprolactone) scaffolds manufactured through BioCell printing: The effect of pore size and geometry on compressive mechanical behaviour and in vitro hMSC viability. Biofabrication 2013, 5, 045004. [Google Scholar] [CrossRef]
- Ostrowska, B.; Di Luca, A.; Szlazak, K.; Moroni, L.; Swieszkowski, W. Influence of internal pore architecture on biological and mechanical properties of three-dimensional fiber deposited scaffolds for bone regeneration. J. Biomed. Mater. Res. A 2016, 104, 991–1001. [Google Scholar] [CrossRef]
- Kumar, S.; Raj, S.; Kolanthai, E.; Sood, A.K.; Sampath, S.; Chatterjee, K. Chemical functionalization of graphene to augment stem cell osteogenesis and inhibit biofilm formation on polymer composites for orthopedic applications. ACS Appl. Mater. Interfaces 2015, 7, 3237–3252. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Chen, B. Poly(ε-caprolactone)/graphene oxide biocomposites: Mechanical properties and bioactivity. Biomed. Mater. 2011, 6, 055010. [Google Scholar] [CrossRef]
- Sayyar, S.; Murray, E.; Thompson, B.C.; Gambhir, S.; Officer, D.L.; Wallace, G.C. Covalently linked biocompatible graphene/polycaprolactone composites for tissue engineering. Carbon 2013, 52, 296–304. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Gao, H.; Zhu, G.; Cao, X.; Shi, X.; Wang, Y. The preparation and characterization of polycaprolactone/graphene oxide biocomposite nanofiber scaffolds and their application for directing cell behaviors. Carbon 2015, 95, 1039–1050. [Google Scholar] [CrossRef]
- Ramazani, S.; Karimi, M. Aligned poly(ε-caprolactone)/graphene oxide and reduced graphene oxide nanocomposite nanofibers: Morphological, mechanical and structural properties. Mater. Sci. Eng. C 2015, 56, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Al Habis, N.; Lafdi, K.; Tsonis, P.A.; Rio-Tsonis, K.D. Enhancing the cell growth using conductive scaffolds. J. Nanomed. Nanotechnol. 2018, 9, 2. [Google Scholar] [CrossRef]
- Shim, J.H.; Won, J.W.; Park, J.H.; Bae, J.H.; Ahn, G.; Kim, C.H.; Lim, D.H.; Cho, D.W.; Yun, W.S.; Bae, E.B.; et al. Effects of 3D-printed polycaprolactone/beta-tricalcium phosphate membranes on guided bone regeneration. Int. J. Mol. Sci. 2017, 18, 899. [Google Scholar] [CrossRef]
- Wang, W.; Caetano, G.F.; Chiang, W.H.; Braz, A.L.; Blaker, J.J.; Frade, M.A.; Bártolo, P.J. Morphological, mechanical and biological assessment of PCL/pristine graphene scaffolds for bone regeneration. Int. J. Bioprint. 2016, 2, 95–105. [Google Scholar] [CrossRef]
- Wang, W.; Caetano, G.; Ambler, W.A.; Blaker, J.J.; Frade, M.A.; Mandal, P.; Diver, C.; Bártolo, P.J. Enhancing the hydrophilicity and cell attachment of 3D printed PCL/graphene scaffolds for bone tissue engineering. Materials 2016, 9, 992. [Google Scholar] [CrossRef]
- Ciesielski, A.; Samori, P. Graphene via sonication assisted liquid-phase exfoliation. Chem. Soc. Rev. 2014, 43, 381–398. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, H.; Xue, Y.; Li, B.; Cai, W. Liquid-Phase exfoliation of graphene: An overview on exfoliation media, techniques, and challenges. Nanomaterials 2018, 8, 942. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Cao, R.; Jiang, S. Morphology, thermal and mechanical properties of poly (ε-caprolactone) biocomposites reinforced with nano-hydroxyapatite decorated graphene. J. Colloid Interface Sci. 2017, 496, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Ege, D.; Cameron, R.; Best, S. The degradation behavior of nanoscale HA/PLGA and α-TCP/PLGA composites. Bioinspir. Biomim. Nanobiomat. 2014, 3, 85–93. [Google Scholar] [CrossRef]
- Huang, J.; Xiong, J.; Liu, J.; Zhu, W.; Wang, D. Investigation of the in vitro degradation of a novel polylactide/nanohydroxyapatite composite for artificial bone. J. Nanomater. 2013, 2013, 515741. [Google Scholar] [CrossRef]
- Duan, J.; Xie, Y.; Yang, J.; Huang, T.; Zhang, N.; Wang, Y.; Zhang, J. Graphene oxide induced hydrolytic degradation behavior changes of poly(L-lactide) in different mediums. Polym. Test. 2016, 56, 220–228. [Google Scholar] [CrossRef]
- Díaz, E.; Sandonis, I.; Valle, M.B. In vitro degradation of poly(caprolactone)/nHA composites. J. Nanomater. 2014, 2014, 802435. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, S.; Diban, N.; Urtiaga, A. Hydrolytic degradation and mechanical stability of Poly(ε--caprolactone)/reduced graphene oxide membranes as scaffolds for in vitro neural tissue regeneration. Membranes 2018, 8, 12. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful in SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Kokubo, T. Formation of biologically active bone-like apatite on metals and polymers by a biomimetic process. Thermochim. Acta 1996, 280–281, 479–490. [Google Scholar] [CrossRef]
- Ataie, A.; Shabani, I.; Seyedjafari, E. Surface mineralized hybrid nanofibrous scaffolds based on poly(L-lactide) and alginate enhances osteogenic differentiation of stem cells. J. Biomed. Mater. Res. A 2019, 107, 586–596. [Google Scholar] [CrossRef]
- De Witte, T.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef]
- Weisgerber, D.W.; Caliari, S.R.; Harley, B.A. Mineralized collagen scaffolds induce hMSC osteogenesis and matrix remodeling. Biomater. Sci. 2015, 3, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Ma, P.X. Porous poly(L-lactic acid)/apatite composites created by biomimetic process. J. Biomed. Mater. Res. 1999, 45, 285–293. [Google Scholar] [CrossRef]
- Chen, Y.; Mak, A.F.; Li, J.; Wang, M.; Shum, A.W. Formation of apatite on poly(α-hydroxy acid) in an accelerated biomimetic process. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 73B, 68–76. [Google Scholar] [CrossRef]
- Rajzer, I.; Menaszek, E.; Kwiatkowski, R.; Chrzanowski, W. Bioactive nanocomposite PLDL/nano-hydroxyapatite electrospun membranes for bone tissue engineering. J. Mater. Sci. Mater. Med. 2014, 25, 1239–1247. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Liu, S.; Li, J.; Guo, K.; Yuan, Q.; Zhong, A.; Yang, J.; Wang, J.; Sun, J.; Wang, Z. Collagen functionalized with graphene oxide enhanced biomimetic mineralization and in situ bone defect repair. ACS Appl. Mater. Interfaces 2018, 10, 44080–44091. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Stenslokken, K.O.; Vaage, I.J. Osteoblast differentiation at a glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef]
- Leong, D.T.; Gupta, A.; Bai, H.F.; Wan, G.; Yoon, L.F.; Too, H.P.; Chew, F.T.; Hutmacher, D.W. Absolute quantification of gene expression in biomaterials research using real-time PCR. Biomaterials 2007, 28, 203–210. [Google Scholar] [CrossRef]
- Van Guilder, H.D.; Vrana, K.E.; Freeman, W.M. Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques 2008, 44, 619–626. [Google Scholar] [CrossRef] [Green Version]
- Serguinko, A.; Wang, M.Y.; Myklebost, O. Real-time vital mineralization detection and quantification during in vitro osteoblast differentiation. Biol. Proced. Online 2018, 20, 14. [Google Scholar] [CrossRef]
- Lock, J.; Nguyen, T.Y.; Liu, H. Nanophase hydroxyapatite and poly(lactide-co-glycolide) composites promote human mesenchymal stem cell adhesion and osteogenic differentiation in vitro. J. Mater. Sci. Mater. Med. 2012, 23, 2543–2552. [Google Scholar] [CrossRef]
- Van Tonder, A.; Joubert, A.M.; Cromarty, A.M. Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res Notes 2015, 8, 47. [Google Scholar] [CrossRef]
- Kenry; Lee, W.C.; Loh, K.P.; Lim, C.T. When stem cells meet graphene: Opportunities and challenges in regenerative medicine. Biomaterials 2018, 155, 230–250. [Google Scholar] [CrossRef]
- Heinrich, D.; Bruland, O.; Guise, T.A.; Suzuki, H.; Sartor, O. Alkaline phosphatase in metastatic castration-resistant prostate cancer: Reassessment of an older biomarker. Future Oncol. 2018, 14, 2543–2556. [Google Scholar] [CrossRef]
- Zhang, Y.; Khan, D.; Delling, J.; Tobiasch, E. Mechanisms underlying the osteo- and adipo-differentiation of human mesenchymal stem cells. Sci. World J. 2012, 2012, 793823. [Google Scholar] [CrossRef]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J. Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue Eng. Part B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef]
- Sun, L.; Pereira, D.; Wang, Q.; Barata, D.B.; Truckenmuller, R.; Li, Z.; Xu, X. Controlling growth and osteogenic differentiation of osteoblasts on microgrooved polystyrene surfaces. PLoS ONE 2016, 11, e0161466. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, Y.G.; Perk, J.W.; Lee, J.M.; Suh, J.Y. The effects of dexamethasone on the apoptosis and osteogenic differentiation of human periodontal ligament cells. J. Periodontal Implant Sci. 2013, 43, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Lagenbach, G.; Handschel, J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef]
- Fu, C.; Bai, H.; Hu, Q.; Gao, T.; Bai, Y. Enhanced proliferation and osteogenic differentiation of MC3T3-E1 pre-osteoblasts on graphene oxide-impregnated PLGA–gelatin nanocomposite fibrous membranes. RSC Adv. 2017, 7, 8886–8897. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Li, Z.; Hou, Y.; Fang, W. Potential mechanisms underlying the Runx2 induced osteogenesis of bone marrow mesenchymal stem cells. Am. J. Transl. Res. 2015, 7, 2527–2535. [Google Scholar]
- Huang, Z.; Nelson, E.R.; Smith, R.L.; Goodman, S.B. The Sequential expression profiles of growth factors from osteroprogenitors to osteoblasts in vitro. Tissue Eng. 2007, 13, 2311–2320. [Google Scholar] [CrossRef]
- Birmingham, E.; Neibur, G.L.; McHugh, P.E.; Shaw, G.; Barry, F.P.; McNamara, L.M. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur. Cell Mater. 2012, 23, 1327. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed Mater Res. 2001, 55, 203–216. [Google Scholar] [CrossRef]
- Rai, B.; Lin, J.L.; Lim, Z.X.H.; Guldberg, R.E.; Hutmacher, D.W.; Cool, S.M. Differences between in vitro viability and differentiation and in vivo bone-forming efficacy of human mesenchymal stem cells cultured on PCL-TCP scaffolds. Biomaterials 2010, 31, 7960–7970. [Google Scholar] [CrossRef]
- Ho, S.T.; Hutmacher, D.W.; Ekaputra, A.K.; Hitendra, D.; Hui, J.H. The evaluation of a biphasic osteochondral implant coupled with an electrospun membrane in a large animal model. Tissue Eng. Part A 2010, 16, 1123–1141. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Zhang, H.Z.; Zhang, Z.Y. 3D printed poly(ε-caprolactone) scaffolds function with simvastatin-loaded poly(lactic-co-glycolic acid) microspheres to repair load-bearing segmental bone defects. Exp. Ther. Med. 2019, 17, 79–90. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Huang, T.; Can, V.M.; Ho, A.S.; Nguyen, S.H.; Nguyen, T.T.T.; Pham, T.N.; Nguyen, T.P.; Nguyen, T.L.; Thi, M.T. In vitro and in vivo tests of PLA/d-Hap nanocomposite. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 045013. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Jia, G.; Jiang, Y.; Liu, Q.; Yang, X.; Pan, S. Improving osteogenesis of PLGA/HA porous scaffolds based on dual delivery of BMP-2 and IGF-1 via a polydopamine coating. RSC Adv. 2017, 7, 56732–256742. [Google Scholar] [CrossRef]
- Zhou, T.; Li, G.; Lin, S.; Tian, T.; Ma, Q.; Zhang, Q.; Shi, S.; Xue, C.; Ma, W.; Cai, X.; et al. Electrospun poly (3-hydroxybutyrate-co-4-hydroxybutyrate)/graphene oxide scaffold: Enhanced properties and promoted in vivo bone repair in rats. ACS Appl. Mater. Interfaces 2017, 9, 42589–42600. [Google Scholar] [CrossRef]
- Peng, S.; Feng, P.; Wu, P.; Huang, W.; Yang, Y.; Guo, W.; Gao, C.; Shuai, C. Graphene oxide as an interface phase between polyetheretherketone and hydroxyapatite for tissue engineering scaffolds. Sci. Rep. 2017, 7, 46604. [Google Scholar] [CrossRef]
- Zhang, B.; Wei, P.; Zhou, Z.; We, T. Interactions of graphene with mammalian cells: Molecular mechanisms and biomedical insights. Adv. Drug Del. Rev. 2016, 105 B, 145–162. [Google Scholar] [CrossRef]
- Ali-Boucetta, H.; Bitounis, D.; Raveendran-Nair, R.; Servant, A.; Van den Bossche, J.; Kostarelo, K. Purified graphene oxide dispersions lack in vitro cytotoxicity and in vivo pathogenicity. Adv. Health Mater. 2013, 2, 433–441. [Google Scholar] [CrossRef]
- Yang, K.; Gong, H.; Shi, X.; Wan, J.; Zhang, Y.; Liu, Z. In vivo biodistribution and toxicology of functionalizednano-graphene oxide in mice after oral and intraperitoneal administration. Biomaterials 2013, 34, 2787–2795. [Google Scholar] [CrossRef]
- Ma, J.; Liu, R.; Wang, X.; Liu, Q.; Chen, Y.; Valle, R.P.; Zuo, Y.; Xia, T.; Liu, X. Crucial role of lateral size for graphene oxide in activating macrophages and stimulating pro-inflammatory responses in cells and animals. ACS Nano 2015, 9, 10498–10515. [Google Scholar] [CrossRef]
- Li, R.; Guiney, L.M.; Chang, C.H.; Mansukani, N.D.; Ji, Z.; Wang, X.; Liao, Y.P.; Jiang, W.; Sun, B.; Hersam, M.C.; et al. Surface oxidation of graphene oxide determines membrane damage, lipid peroxidation, and cytotoxicity in macrophages in a pulmonary toxicity model. ACS Nano 2018, 12, 1390–1402. [Google Scholar] [CrossRef]
- Mendonca, M.C.; Soares, E.S.; de Jesus, M.B.; Ceragioli, H.J.; Ferreira, M.S.; Catharino, R.R.; Cruz-Hofling, M.A. Reduced graphene oxide induces transient blood–brain barrier opening: An in vivo study. J. Nanobiotechnol. 2015, 13, 78. [Google Scholar] [CrossRef]
- Katayama, I.; Miyaji, H.; Takita, H.; Nishida, E.; Tsuji, M.; Fugetsu, B.; Sun, L.; Inoue, K.; Ibara, A.; Akasaka, T.; et al. Comparative study of bioactivity of collagen scaffolds coated with graphene oxide and reduced graphene oxide. Int. J. Nanomedic. 2014, 9, 3363–3373. [Google Scholar] [CrossRef] [Green Version]
- Sarker, M.D.; Naghieh, S.; Sharma, N.K.; Chen, X. 3D biofabrication of vascular networks for tissue regeneration: A report on recent advances. J. Pharm. Anal. 2018, 8, 277–296. [Google Scholar] [CrossRef]






































| Specimen Composition, wt% | Fabrication Route | Porosity, % | Pore Size, µm | Fiber Diameter, nm | Mechanical Test Method | Modulus, MPa | Strength, MPa | Elongation, % | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PLA-Based Scaffolds | |||||||||
| PLA | SC/PL | 92.0 | NA | None | Compression | 4.7 | 0.29 | NA | [61] |
| PLA/10% nHA | SC/PL | 91.4 | NA | None | Compression | 5.0 | 0.32 | NA | [61] |
| PLA/20% nHA | SC/PL | 90.6 | NA | None | Compression | 6.4 | 0.35 | NA | [61] |
| PLA/50% nHA | SC/PL | 86.5 | NA | None | Compression | 9.8 | 0.44 | NA | [61] |
| PLA | TIPS | 93.0 | 50–100 | None | Compression | 4.3 | NA | NA | [60] |
| PLA/10% nHA | TIPS | 92.8 | 50–100 | None | Compression | 4.9 | NA | NA | [60] |
| PLA/30% nHA | TIPS | 92.3 | 50–100 | None | Compression | 7.8 | NA | NA | [60] |
| PLA/50% nHA | TIPS | 91.8 | 50–100 | None | Compression | 8.3 | NA | NA | [60] |
| PLA | TIPS | 87.4 | 175 | None | Compression | 2.4 | 1.79 | NA | [179] |
| PLA/50% nHA | TIPS | 85.1 | 175 | None | Compression | 14.9 | 8.67 | NA | [179] |
| PLA | ES | NA | 4.51 | 365 | Tension | 0.42 | 0.063 | 27 | [182] |
| PLA/5% nHA | ES | NA | 1.32 | 255 | Tension | 1.80 | 0.157 | 30 | [182] |
| PLA/20% nHA | ES | NA | 0.53 | 135 | Tension | 4.71 | 0.262 | 36 | [182] |
| PLA | LAM | 55.3 | 271 | None | Tension; Bending | 2.8 (B) | 42.5 (T); 122.8 (B) | NA | [190] |
| PLA/10% nHA | LAM | 68.5 | 336 | None | Tension; Bending | 3.1 (B) | 38.6 (T); 131.9 (B) | NA | [190] |
| PLA/20% nHA | LAM | 85.1 | 392 | None | Tension; Bending | 3.5 (B) | 35.1 (T); 138.6 (B) | NA | [190] |
| PLA/30% nHA | LAM | 76.3 | 354 | None | Tension; Bending | 3.8 (B) | 29.2 (T); 119.1 (B) | NA | [190] |
| PLA/40% nHA | LAM | 72.2 | 318 | None | Tension; Bending | 3.9 (B) | 23.2 (T); 112.5 (B) | NA | [190] |
| PLA | ES | NA | NA | 839 | Tension | NA | 2.1 | 97 | [200] |
| PLA/1% GO | ES | NA | NA | 706 | Tension | NA | 2.9 | 85 | [200] |
| PLA/2% GO | ES | NA | NA | 863 | Tension | NA | 3.5 | 87 | [200] |
| PLA/1% GO-g-PEG | ES | NA | NA | 593 | Tension | NA | 3.9 | 95 | [200] |
| PLA/2% GO-g-PEG | ES | NA | NA | 761 | Tension | NA | 4.5 | 83 | [200] |
| PLA/5% GO-g-PEG | ES | NA | NA | 685 | Tension | NA | 5.2 | 84 | [200] |
| PLA | ES | 70.5 | NA | 786 | Tension | 8.58 | 0.27 | NA | [81] |
| PLA/15% nHA | ES | 74.5 | NA | 563 | Tension | 9.88 | 0.41 | NA | [81] |
| PLA/15% nHA-1% GO | ES | 75.6 | NA | 516 | Tension | 12.69 | 0.47 | NA | [81] |
| PLA/15% nHA-2% GO | ES | 76.2 | NA | 502 | Tension | 16.73 | 0.57 | NA | [81] |
| PLA/15% nHA-3% GO | ES | 77.9 | NA | 412 | Tension | 8.10 | 0.38 | NA | [81] |
| PCL-Based Scaffolds | |||||||||
| PCL | ES | NA | 1.94 | 393 | Tension | NA | 12.3 | 380 | [213] |
| PCL/30% nHA | ES | NA | 2.19 | 317 | Tension | NA | 85.17 | 530 | [213] |
| PCL/60% nHA | ES | NA | 2.30 | 332 | Tension | NA | 158.1 | 564 | [213] |
| PCL | SLS | 63.1 | 1750 × 1750 | NA | Compression | 65 | 3.2 | NA | [214] |
| PCL | SLS | 79.0 | 2000 × 2500 | NA | Compression | 54 | 2.0 | NA | [214] |
| PCL | SLS | 78.54 | NA | NA | Compression | NA | 1.38 | NA | [215] |
| PCL/10% nHA | SLS | 72.06 | NA | NA | Compression | NA | 2.67 | NA | [215] |
| PCL/15% nHA | SLS | 70.31 | NA | NA | Compression | NA | 3.17 | NA | [215] |
| PCL | ES | 94.1 | NA | 543 | Tension | 10.5 | 2.37 | 110 | [228] |
| PCL/0.3% GO | ES | 94.6 | NA | 640 | Tension | 17.4 | 4.61 | 275 | [228] |
| Trabecular bone; dried defatted | NA | NA | NA | NA | Compression | 1.1–139 | 0.3–7.0 | NA | [15] |
| Trabecular bone; fresh frozen | NA | NA | NA | NA | Compression | 1.4–79 | 1.5–45 | NA | [15] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liao, C.; Tjong, S.C. Synthetic Biodegradable Aliphatic Polyester Nanocomposites Reinforced with Nanohydroxyapatite and/or Graphene Oxide for Bone Tissue Engineering Applications. Nanomaterials 2019, 9, 590. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9040590
Li Y, Liao C, Tjong SC. Synthetic Biodegradable Aliphatic Polyester Nanocomposites Reinforced with Nanohydroxyapatite and/or Graphene Oxide for Bone Tissue Engineering Applications. Nanomaterials. 2019; 9(4):590. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9040590
Chicago/Turabian StyleLi, Yuchao, Chengzhu Liao, and Sie Chin Tjong. 2019. "Synthetic Biodegradable Aliphatic Polyester Nanocomposites Reinforced with Nanohydroxyapatite and/or Graphene Oxide for Bone Tissue Engineering Applications" Nanomaterials 9, no. 4: 590. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9040590