Influence of the Ce4+/Ce3+ Redox-Couple on the Cyclic Regeneration for Adsorptive and Catalytic Performance of NiO-PdO/CeO2±δ Nanoparticles for n-C7 Asphaltene Steam Gasification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Selection of Catalytic Nanoparticles
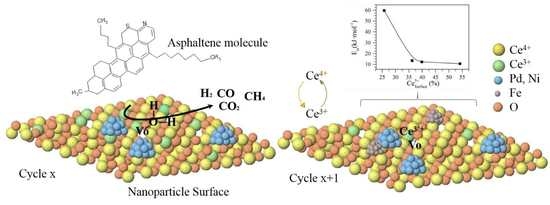
2.2.2. Nanoparticles Regeneration
2.2.3. The n-C7 Asphaltene Adsorption over Nanoparticles
2.2.4. Thermogravimetric Analyses
2.2.5. X-ray Photoelectron Spectroscopy Analysis
3. Modeling
3.1. Double Exponential Model
3.2. Solid−Liquid Equilibrium (SLE) Model
3.3. Thermodynamic Properties of Adsorption
3.4. Adsorption Potential Model by Michael Polanyi
3.5. Estimation of Effective Activation Energy
3.6. Statistical Analysis
4. Results and Discussion
4.1. Selection of Nanocatalyst
4.2. Adsorption Kinetics
4.3. Adsorption Isotherms
4.4. Thermodynamic Studies
4.5. Polanyi’s Adsorption Potential
4.6. Thermogravimetric Analysis of n-C7 Asphaltenes
4.6.1. Mass Loss Analysis
4.6.2. Isothermal Conversion
4.6.3. Effective Activation Energy of n-C7 Asphaltene Thermo-Decomposition in the Presence and Absence of Nanoparticles
4.7. XPS Analysis of CeNi0.89Pd1.1 Nanoparticles through Catalytic Regeneration Cycles
4.7.1. Catalytic Effect of Ce4+/Ce3+ Redox Couple on the Thermodynamic and Adsorption Properties of CeNi0.89Pd1.1 Nanoparticles
4.7.2. Effective Activation Energy and Kinetics of the Catalytic Steam Gasification of Asphaltenes in the Presence and Absence of Nanoparticles
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shah, A.; Fishwick, R.; Wood, J.; Leeke, G.; Rigby, S.; Greaves, M. A review of novel techniques for heavy oil and bitumen extraction and upgrading. Energy Environ. Sci. 2010, 3, 700–714. [Google Scholar] [CrossRef]
- International Energy Agency. World Energy Outlook 2017; International Energy Agency: Paris, France, 2017. [Google Scholar]
- Santos, R.; Loh, W.; Bannwart, A.; Trevisan, O. An overview of heavy oil properties and its recovery and transportation methods. Braz. J. Chem. Eng. 2014, 31, 571–590. [Google Scholar] [CrossRef] [Green Version]
- Guo, K.; Li, H.; Yu, Z. In-situ heavy and extra-heavy oil recovery: A review. Fuel 2016, 185, 886–902. [Google Scholar] [CrossRef]
- Curtis, C.; Kopper, R.; Decoster, E.; Guzmán-Garcia, A.; Huggins, C.; Knauer, L.; Minner, M.; Kupsch, N.; Linares, L.M.; Rough, H. Heavy-oil reservoirs. Oilfield Rev. 2002, 14, 30–51. [Google Scholar]
- Rahimi, P.M.; Gentzis, T. The chemistry of bitumen and heavy oil processing. In Practical Advances in Petroleum Processing; Springer: Devon, AB, Canada, 2006; pp. 597–634. [Google Scholar]
- Hamedi Shokrlu, Y.; Babadagli, T. Kinetics of the in-situ upgrading of heavy oil by nickel nanoparticle catalysts and its effect on cyclic-steam-stimulation recovery factor. SPE Reserv. Eval. Eng. 2014, 17, 355–364. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Metal oxide nanoparticles for asphaltene adsorption and oxidation. Energy Fuels 2011, 25, 1017–1023. [Google Scholar] [CrossRef]
- Buckley, J.S.; Liu, Y.; Xie, X.; Morrow, N.R. Asphaltenes and crude oil wetting-the effect of oil composition. SPE J. 1997, 2, 107–119. [Google Scholar] [CrossRef]
- Terry, R.E. Enhanced oil recovery. Encycl. Phys. Sci. Technol. 2001, 18, 503–518. [Google Scholar]
- Alvarado, V.; Manrique, E. Enhanced oil recovery: An update review. Energies 2010, 3, 1529–1575. [Google Scholar] [CrossRef]
- Thomas, S. Enhanced oil recovery—An overview. Oil Gas Sci. Technol. Revue de l’IFP 2008, 63, 9–19. [Google Scholar] [CrossRef]
- Cardona, L.; Arias-Madrid, D.; Cortés, F.; Lopera, S.; Franco, C. Heavy oil upgrading and enhanced recovery in a steam injection process assisted by NiO-and PdO-Functionalized SiO2 nanoparticulated catalysts. Catalysts 2018, 8, 132. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, S.; Zhang, L.; Peng, X.; Pei, S.; Cui, G.; Liu, Y. Numerical study of air assisted cyclic steam stimulation process for heavy oil reservoirs: Recovery performance and energy efficiency analysis. Fuel 2018, 211, 471–483. [Google Scholar] [CrossRef]
- Chaar, M.; Venetos, M.; Dargin, J.; Palmer, D. Economics of Steam Generation for Thermal Enhanced Oil Recovery. Oil Gas Facil. 2015, 4, 42–50. [Google Scholar] [CrossRef]
- Ali, S.; Thomas, S. The promise and problems of enhanced oil recovery methods. J. Can. Pet. Technol. 1996, 35. [Google Scholar] [CrossRef]
- Karacan, C.Ö.; Okandan, E. Change of physical and thermal decomposition properties of in situ heavy oil with steam temperature. Pet. Sci. Technol. 1997, 15, 429–443. [Google Scholar] [CrossRef]
- Kowalewski, I.; Fiedler, C.; Parra, T.; Adam, P.; Albrecht, P. Preliminary results on the formation of organosulfur compounds in sulfate-rich petroleum reservoirs submitted to steam injection. Org. Geochem. 2008, 39, 1130–1136. [Google Scholar] [CrossRef]
- Kar, T.; Ovalles, C.; Rogel, E.; Vien, J.; Hascakir, B. The residual oil saturation determination for Steam Assisted Gravity Drainage (SAGD) and Solvent-SAGD. Fuel 2016, 172, 187–195. [Google Scholar] [CrossRef]
- Nassar, N.N.; Franco, C.A.; Montoya, T.; Cortés, F.B.; Hassan, A. Effect of oxide support on Ni–Pd bimetallic nanocatalysts for steam gasification of n-C7 asphaltenes. Fuel 2015, 156, 110–120. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Application of nanotechnology for heavy oil upgrading: Catalytic steam gasification/cracking of asphaltenes. Energy Fuels 2011, 25, 1566–1570. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Chen, G.; Gai, Z. Application of nanoparticles in enhanced oil recovery: A critical review of recent progress. Energies 2017, 10, 345. [Google Scholar] [CrossRef]
- Agista, M.N.; Guo, K.; Yu, Z. A State-of-the-Art Review of Nanoparticles Application in Petroleum with a Focus on Enhanced Oil Recovery. Appl. Sci. 2018, 8, 871. [Google Scholar] [CrossRef]
- Hashemi, R.; Nassar, N.N.; Almao, P.P. Nanoparticle technology for heavy oil in-situ upgrading and recovery enhancement: Opportunities and challenges. Appl. Energy 2014, 133, 374–387. [Google Scholar] [CrossRef]
- Cheraghian, G.; Hendraningrat, L. A review on applications of nanotechnology in the enhanced oil recovery part B: Effects of nanoparticles on flooding. Int. Nano Lett. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Franco, C.A.; Montoya, T.; Nassar, N.N.; Pereira-Almao, P.; Cortés, F.B. Adsorption and subsequent oxidation of colombian asphaltenes onto nickel and/or palladium oxide supported on fumed silica nanoparticles. Energy Fuels 2013, 27, 7336–7347. [Google Scholar] [CrossRef]
- López, D.; Giraldo, L.J.; Salazar, J.P.; Zapata, D.M.; Ortega, D.C.; Franco, C.A.; Cortés, F.B. Metal Oxide Nanoparticles Supported on Macro-Mesoporous Aluminosilicates for Catalytic Steam Gasification of Heavy Oil Fractions for On-Site Upgrading. Catalysts 2017, 7, 319. [Google Scholar] [CrossRef]
- Hosseinpour, N.; Mortazavi, Y.; Bahramian, A.; Khodatars, L.; Khodadadi, A.A. Enhanced pyrolysis and oxidation of asphaltenes adsorbed onto transition metal oxides nanoparticles towards advanced in-situ combustion EOR processes by nanotechnology. Appl. Catal. A Gen. 2014, 477, 159–171. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Comparative oxidation of adsorbed asphaltenes onto transition metal oxide nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 145–149. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Carbognani, L.; Lopez-Linares, F.; Pereira-Almao, P. Iron oxide nanoparticles for rapid adsorption and enhanced catalytic oxidation of thermally cracked asphaltenes. Fuel 2012, 95, 257–262. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Effect of the particle size on asphaltene adsorption and catalytic oxidation onto alumina particles. Energy Fuels 2011, 25, 3961–3965. [Google Scholar] [CrossRef]
- Druetta, P.; Raffa, P.; Picchioni, F. Plenty of Room at the Bottom: Nanotechnology as Solution to an Old Issue in Enhanced Oil Recovery. Appl. Sci. 2018, 8, 2596. [Google Scholar] [CrossRef]
- Hassan, A.; Lopez-Linares, F.; Nassar, N.N.; Carbognani-Arambarri, L.; Pereira-Almao, P. Development of a support for a NiO catalyst for selective adsorption and post-adsorption catalytic steam gasification of thermally converted asphaltenes. Catal. Today 2013, 207, 112–118. [Google Scholar] [CrossRef]
- Sehested, J.; Gelten, J.A.; Remediakis, I.N.; Bengaard, H.; Nørskov, J.K. Sintering of nickel steam-reforming catalysts: Effects of temperature and steam and hydrogen pressures. J. Catal. 2004, 223, 432–443. [Google Scholar] [CrossRef]
- Nezhad, S.S.K.; Cheraghian, G. Mechanisms behind injecting the combination of nano-clay particles and polymer solution for enhanced oil recovery. Appl. Nanosci. 2016, 6, 923–931. [Google Scholar] [CrossRef]
- Cheraghian, G.; Nezhad, S.S.K.; Kamari, M.; Hemmati, M.; Masihi, M.; Bazgir, S. Adsorption polymer on reservoir rock and role of the nanoparticles, clay and SiO2. Int. Nano Lett. 2014, 4, 114. [Google Scholar] [CrossRef]
- Cheraghian, G. Synthesis and properties of polyacrylamide by nanoparticles, effect nanoclay on stability polyacrylamide solution. Micro Nano Lett. 2017, 12, 40–44. [Google Scholar] [CrossRef]
- Cheraghian, G. Evaluation of clay and fumed silica nanoparticles on adsorption of surfactant polymer during enhanced oil recovery. J. Jpn. Pet. Inst. 2017, 60, 85–94. [Google Scholar] [CrossRef]
- Cardona Rojas, L. Efecto de nanopartículas en procesos con inyección de vapor a diferentes calidades. Master’s Thesis, Universidad Nacional de Colombia-Sede Medellín, Medellín, Colombia, March 2018. [Google Scholar]
- Mdina, O.E.; Gallego, J.; Arias-Madrid, D.; Cortés, F.B.; Franco, C.A. Optimization of the Load of Transition Metal Oxides (Fe2O3, Co3O4, NiO and/or PdO) onto CeO2 Nanoparticles in Catalytic Steam Decomposition of n-C7 Asphaltenes at Low Temperatures. Nanomaterials 2019, 9, 401. [Google Scholar] [CrossRef]
- Atta, A.; Al-Lohedan, H.; Al-Hussain, S. Functionalization of magnetite nanoparticles as oil spill collector. Int. J. Mol. Sci. 2015, 16, 6911–6931. [Google Scholar] [CrossRef]
- Guo, K.; Zhang, Y.; Shi, Q.; Yu, Z. The effect of carbon-supported nickel nanoparticles in the reduction of carboxylic acids for in situ upgrading of heavy crude oil. Energy Fuels 2017, 31, 6045–6055. [Google Scholar] [CrossRef]
- Hosseinpour, M.; Fatemi, S.; Ahmadi, S.J. Catalytic cracking of petroleum vacuum residue in supercritical water media: Impact of α-Fe2O3 in the form of free nanoparticles and silica-supported granules. Fuel 2015, 159, 538–549. [Google Scholar] [CrossRef]
- Hosseinpour, N.; Khodadadi, A.A.; Bahramian, A.; Mortazavi, Y. Asphaltene adsorption onto acidic/basic metal oxide nanoparticles toward in situ upgrading of reservoir oils by nanotechnology. Langmuir 2013, 29, 14135–14146. [Google Scholar] [CrossRef]
- Kazemzadeh, Y.; Eshraghi, S.E.; Kazemi, K.; Sourani, S.; Mehrabi, M.; Ahmadi, Y. Behavior of asphaltene adsorption onto the metal oxide nanoparticle surface and its effect on heavy oil recovery. Ind. Eng. Chem. Res. 2015, 54, 233–239. [Google Scholar] [CrossRef]
- Li, L.; Zhan, Y.; Zheng, Q.; Zheng, Y.; Lin, X.; Li, D.; Zhu, J. Water–Gas Shift Reaction Over Aluminum Promoted Cu/CeO2 Nanocatalysts Characterized by XRD, BET, TPR and Cyclic Voltammetry (CV). Catal. Lett. 2007, 118, 91–97. [Google Scholar] [CrossRef]
- Nematollahi, B.; Rezaei, M.; Lay, E.N. Preparation of highly active and stable NiO–CeO2 nanocatalysts for CO selective methanation. Int. J. Hydrog. Energy 2015, 40, 8539–8547. [Google Scholar] [CrossRef]
- Khajenoori, M.; Rezaei, M.; Nematollahi, B. Preparation of noble metal nanocatalysts and their applications in catalytic partial oxidation of methane. J. Ind. Eng. Chem. 2013, 19, 981–986. [Google Scholar] [CrossRef]
- Estifaee, P.; Haghighi, M.; Mohammadi, N.; Rahmani, F. CO oxidation over sonochemically synthesized Pd–Cu/Al2O3 nanocatalyst used in hydrogen purification: Effect of Pd loading and ultrasound irradiation time. Ultrason. Sonochem. 2014, 21, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- De Lasa, H.; Salaices, E.; Mazumder, J.; Lucky, R. Catalytic steam gasification of biomass: Catalysts, thermodynamics and kinetics. Chem. Rev. 2011, 111, 5404–5433. [Google Scholar] [CrossRef]
- Koh, A.C.; Leong, W.K.; Chen, L.; Ang, T.P.; Lin, J.; Johnson, B.F.; Khimyak, T. Highly efficient ruthenium and ruthenium–platinum cluster-derived nanocatalysts for hydrogen production via ethanol steam reforming. Catal. Commun. 2008, 9, 170–175. [Google Scholar] [CrossRef]
- Vidal, H.; Kašpar, J.; Pijolat, M.; Colon, G.; Bernal, S.; Cordón, A.; Perrichon, V.; Fally, F. Redox behavior of CeO2–ZrO2 mixed oxides: I. Influence of redox treatments on high surface area catalysts. Appl. Catal. B Environ. 2000, 27, 49–63. [Google Scholar] [CrossRef]
- Wang, R.; Xu, H.; Liu, X.; Ge, Q.; Li, W. Role of redox couples of Rh0/Rhδ+ and Ce4+/Ce3+ in CH4/CO2 reforming over Rh–CeO2/Al2O3 catalyst. Appl. Catal. A Gen. 2006, 305, 204–210. [Google Scholar] [CrossRef]
- Celardo, I.; Traversa, E.; Ghibelli, L. Cerium oxide nanoparticles: A promise for applications in therapy. J. Exp. Ther. Oncol. 2011, 9, 47–51. [Google Scholar]
- Das, M.; Patil, S.; Bhargava, N.; Kang, J.-F.; Riedel, L.M.; Seal, S.; Hickman, J.J. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials 2007, 28, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Hayek, K.; Kramer, R.; Paál, Z. Metal-support boundary sites in catalysis. Appl. Catal. A Gen. 1997, 162, 1–15. [Google Scholar] [CrossRef]
- Alamolhoda, S.; Vitale, G.; Hassan, A.; Nassar, N.N.; Almao, P.P. Synergetic effects of cerium and nickel in Ce-Ni-MFI catalysts on low-temperature water-gas shift reaction. Fuel 2019, 237, 361–372. [Google Scholar] [CrossRef]
- Munnik, P.; de Jongh, P.E.; de Jong, K.P. Recent developments in the synthesis of supported catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef]
- Lensveld, D.J.; Mesu, J.G.; van Dillen, A.J.; de Jong, K.P. The application of well-dispersed nickel nanoparticles inside the mesopores of MCM-41 by use of a nickel citrate chelate as precursor. Stud. Surf. Sci. Catal. 2000, 143, 647–657. [Google Scholar]
- Mansfield, E.; Tyner, K.M.; Poling, C.M.; Blacklock, J.L. Determination of nanoparticle surface coatings and nanoparticle purity using microscale thermogravimetric analysis. Anal. Chem. 2014, 86, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Lv, L.; Pan, B.-C.; Zhang, Q.-J.; Zhang, W.-M.; Zhang, Q.-X. Critical review in adsorption kinetic models. J. Zhejiang Univ. Sci. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Chiron, N.; Guilet, R.; Deydier, E. Adsorption of Cu(II) and Pb(II) onto a grafted silica: Isotherms and kinetic models. Water Res. 2003, 37, 3079–3086. [Google Scholar] [CrossRef]
- Wilczak, A.; Keinath, T.M. Kinetics of sorption and desorption of copper (II) and lead (II) on activated carbon. Water Environ. Res. 1993, 65, 238–244. [Google Scholar] [CrossRef]
- Talu, O.; Meunier, F. Adsorption of associating molecules in micropores and application to water on carbon. AIChE J. 1996, 42, 809–819. [Google Scholar] [CrossRef]
- Montoya, T.; Coral, D.; Franco, C.A.; Nassar, N.N.; Cortés, F.B. A novel solid–liquid equilibrium model for describing the adsorption of associating asphaltene molecules onto solid surfaces based on the “chemical theory”. Energy Fuels 2014, 28, 4963–4975. [Google Scholar] [CrossRef]
- Nassar, N.N. Asphaltene adsorption onto alumina nanoparticles: Kinetics and thermodynamic studies. Energy Fuels 2010, 24, 4116–4122. [Google Scholar] [CrossRef]
- Cortés, F.B.; Montoya, T.; Acevedo, S.; Nassar, N.N.; Franco, C.A. Adsorption-desorption of n-c7 asphaltenes over micro-and nanoparticles of silica and its impact on wettability alteration. CT F-Cienc. Tecnol. Futuro 2016, 6, 89–106. [Google Scholar] [CrossRef]
- Betancur, S.; Carrasco-Marín, F.; Franco, C.A.; Cortés, F.B. Development of Composite Materials Based on the Interaction between Nanoparticles and Surfactants for Application on Chemical Enhanced Oil Recovery. Ind. Eng. Chem. Res. 2018, 57, 2367–12377. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Luna, G.; Pereira-Almao, P. Kinetics of the catalytic thermo-oxidation of asphaltenes at isothermal conditions on different metal oxide nanoparticle surfaces. Catal. Today 2013, 207, 127–132. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Scheffe, H. The simplex-centroid design for experiments with mixtures. J. R. Stat. Soc. Ser. B 1963, 235–263. [Google Scholar] [CrossRef]
- Murugan, P.; Mahinpey, N.; Mani, T. Thermal cracking and combustion kinetics of asphaltenes derived from Fosterton oil. Fuel Process. Technol. 2009, 90, 1286–1291. [Google Scholar] [CrossRef]
- Mullins, O.C.; Sabbah, H.; Eyssautier, J.; Pomerantz, A.E.; Barré, L.; Andrews, A.B.; Ruiz-Morales, Y.; Mostowfi, F.; McFarlane, R.; Goual, L. Advances in asphaltene science and the Yen–Mullins model. Energy Fuels 2012, 26, 3986–4003. [Google Scholar] [CrossRef]
- Nassar, N.N.; Betancur, S.; Acevedo, S.c.; Franco, C.A.; Cortés, F.B. Development of a population balance model to describe the influence of shear and nanoparticles on the aggregation and fragmentation of asphaltene aggregates. Ind. Eng. Chem. Res. 2015, 54, 8201–8211. [Google Scholar] [CrossRef]
- Franco, C.; Patiño, E.; Benjumea, P.; Ruiz, M.A.; Cortés, F.B. Kinetic and thermodynamic equilibrium of asphaltenes sorption onto nanoparticles of nickel oxide supported on nanoparticulated alumina. Fuel 2013, 105, 408–414. [Google Scholar] [CrossRef]
- Wu, S.; Tang, D.; Li, S.; Chen, H.; Wu, H. Coalbed methane adsorption behavior and its energy variation features under supercritical pressure and temperature conditions. J. Pet. Sci. Eng. 2016, 146, 726–734. [Google Scholar] [CrossRef]
- Franco, C.A.; Nassar, N.N.; Montoya, T.; Ruíz, M.A.; Cortés, F.B. Influence of asphaltene aggregation on the adsorption and catalytic behavior of nanoparticles. Energy Fuels 2015, 29, 1610–1621. [Google Scholar] [CrossRef]
- Sharma, A.; Saito, I.; Nakagawa, H.; Miura, K. Effect of carbonization temperature on the nickel crystallite size of a Ni/C catalyst for catalytic hydrothermal gasification of organic compounds. Fuel 2007, 86, 915–920. [Google Scholar] [CrossRef]
- Cao, A.; Lu, R.; Veser, G. Stabilizing metal nanoparticles for heterogeneous catalysis. Phys. Chem. Chem. Phys. 2010, 12, 13499–13510. [Google Scholar] [CrossRef] [PubMed]
- Arboleda, J.; Castillo, Á.; Muñoz, S. Estudio de la acuatermólisis catalítica en procesos de upgrading de crudos pesados como método complementario en el recobro térmico de hidrocarburos. Rev. Fuentes 2018, 16, 57–69. [Google Scholar]
- Ospina Gómez, N.A. Evaluación de la aplicación de nanofluidos para mejoramiento in-situ del crudo pesado. Master’s Thesis, Universidad Nacional de Colombia-Sede Medellín, Medellín, Colombia, 2015. [Google Scholar]
- Ternan, M. Catalytic hydrogenation and asphaltene conversion of Athabasca bitumen. Can. J. Chem. Eng. 1983, 61, 689–696. [Google Scholar] [CrossRef]
- Jacobs, G.; Ricote, S.; Graham, U.M.; Patterson, P.M.; Davis, B.H. Low temperature water gas shift: Type and loading of metal impacts forward decomposition of pseudo-stabilized formate over metal/ceria catalysts. Catal. Today 2005, 106, 259–264. [Google Scholar] [CrossRef]
- Vignatti, C.I.; Avila, M.S.; Apesteguia, C.R.; Garetto, T.F. Study of the water-gas shift reaction over Pt supported on CeO2–ZrO2 mixed oxides. Catal. Today 2011, 171, 297–303. [Google Scholar] [CrossRef]
- Speight, J. Petroleum Asphaltenes-Part 1: Asphaltenes, resins and the structure of petroleum. Oil Gas Sci. Technol. 2004, 59, 467–477. [Google Scholar] [CrossRef]
- Fu, Q.; Deng, W.; Saltsburg, H.; Flytzani-Stephanopoulos, M. Activity and stability of low-content gold–cerium oxide catalysts for the water–gas shift reaction. Appl. Catal. B Environ. 2005, 56, 57–68. [Google Scholar] [CrossRef]
- Hilaire, S.; Wang, X.; Luo, T.; Gorte, R.; Wagner, J. A comparative study of water-gas-shift reaction over ceria supported metallic catalysts. Appl. Catal. A Gen. 2001, 215, 271–278. [Google Scholar] [CrossRef]
- Mierczynski, P.; Maniukiewicz, W.; Maniecki, T. Comparative studies of Pd, Ru, Ni, Cu/ZnAl2O4 catalysts for the water gas shift reaction. Open Chem. 2013, 11, 912–919. [Google Scholar] [Green Version]
- Peuckert, M. XPS study on surface and bulk palladium oxide, its thermal stability, and a comparison with other noble metal oxides. J. Phys. Chem. 1985, 89, 2481–2486. [Google Scholar] [CrossRef]
- Basile, F.; Fornasari, G.; Gazzano, M.; Vaccari, A. Thermal evolution and catalytic activity of Pd/Mg/Al mixed oxides obtained from a hydrotalcite-type precursor. Appl. Clay Sci. 2001, 18, 51–57. [Google Scholar] [CrossRef]
- Pinc, W.; Yu, P.; O’Keefe, M.; Fahrenholtz, W. Effect of gelatin additions on the corrosion resistance of cerium based conversion coatings spray deposited on Al 2024-T3. Surf. Coat. Technol. 2009, 23, 3533–3540. [Google Scholar] [CrossRef]
- Shyu, J.; Weber, W.; Gandhi, H. Surface characterization of alumina-supported ceria. J. Phys. Chem. 1988, 92, 4964–4970. [Google Scholar] [CrossRef]
- Abi-aad, E.; Bechara, R.; Grimblot, J.; Aboukais, A. Preparation and characterization of ceria under an oxidizing atmosphere. Thermal analysis, XPS, and EPR study. Chem. Mater. 1993, 5, 793–797. [Google Scholar] [CrossRef]
- Matta, J.; Courcot, D.; Abi-Aad, E.; Aboukais, A. Thermal analysis, EPR and XPS study of vanadyl (IV) oxalate behavior on the ceria surface. J. Therm. Anal. Calorim. 2001, 66, 717–727. [Google Scholar] [CrossRef]
- Qiu, L.; Liu, F.; Zhao, L.; Ma, Y.; Yao, J. Comparative XPS study of surface reduction for nanocrystalline and microcrystalline ceria powder. Appl. Surf. Sci. 2006, 252, 4931–4935. [Google Scholar] [CrossRef]
- Sim, K.S.; Hilaire, L.; Le Normand, F.; Touroude, R.; Paul-Boncour, V.; Percheron-Guegan, A. Catalysis by palladium–rare-earth-metal (REPd 3) intermetallic compounds: Hydrogenation of but-1-ene, buta-1, 3-diene and but-1-yne. J. Chem. Soc. Faraday Trans. 1991, 87, 1453–1460. [Google Scholar] [CrossRef]
- Kotani, A.; Jo, T.; Parlebas, J. Many-body effects in core-level spectroscopy of rare-earth compounds. Adv. Phys. 1988, 37, 37–85. [Google Scholar] [CrossRef]
- Laachir, A.; Perrichon, V.; Badri, A.; Lamotte, J.; Catherine, E.; Lavalley, J.C.; El Fallah, J.; Hilaire, L.; Le Normand, F.; Quéméré, E. Reduction of CeO2 by hydrogen. Magnetic susceptibility and Fourier-transform infrared, ultraviolet and X-ray photoelectron spectroscopy measurements. J. Chem. Soc. Faraday Trans. 1991, 87, 1601–1609. [Google Scholar] [CrossRef]
- Nagai, Y.; Hirabayashi, T.; Dohmae, K.; Takagi, N.; Minami, T.; Shinjoh, H.; Matsumoto, S.i. Sintering inhibition mechanism of platinum supported on ceria-based oxide and Pt-oxide–support interaction. J. Catal. 2006, 242, 103–109. [Google Scholar] [CrossRef]
- Xu, J.; Harmer, J.; Li, G.; Chapman, T.; Collier, P.; Longworth, S.; Tsang, S.C. Size dependent oxygen buffering capacity of ceria nanocrystals. Chem. Commun. 2010, 46, 1887–1889. [Google Scholar] [CrossRef] [PubMed]













| Cycle | ± 0.01 (mg·g−1) | ± 0.01 (mg·g−1) | ± 0.02 (mg·L−1) | ± 0.01 (min−1) | ± 0.02 (mg·L−1) | ± 0.01 (min−1) | |
|---|---|---|---|---|---|---|---|
| 1 | 0.17 | 0.17 | 0.98 | 0.41 | 0.45 | 0.06 | 0.12 |
| 2 | 0.16 | 0.17 | 0.76 | 0.38 | 0.34 | 0.03 | 0.11 |
| 3 | 0.15 | 0.15 | 0.69 | 0.21 | 0.65 | 0.00 | 0.29 |
| 4 | 0.15 | 0.15 | 0.70 | 0.36 | 0.20 | 0.02 | 0.28 |
| 5 | 0.15 | 0.15 | 0.84 | 0.63 | 0.47 | 0.10 | 0.14 |
| 6 | 0.14 | 0.14 | 0.59 | 0.24 | 0.97 | 0.01 | 0.17 |
| 7 | 0.14 | 0.13 | 0.79 | 0.34 | 0.73 | 0.10 | 0.13 |
| 8 | 0.12 | 0.12 | 0.83 | 0.22 | 0.12 | 0.02 | 0.07 |
| 9 | 0.12 | 0.12 | 0.65 | 0.20 | 0.53 | 0.00 | 0.28 |
| Cycle | Temperature | [mg·g−1] × 10−2 | [g·g−1] × 10−2 | [g·g−1] × 10−2 | |
|---|---|---|---|---|---|
| 25 | 2.64 | 1.15 | 27.03 | 0.004 | |
| 1 | 55 | 8.45 | 3.35 | 28.86 | 0.014 |
| 75 | 15.98 | 3.45 | 29.68 | 0.023 | |
| 25 | 2.65 | 1.15 | 27.02 | 0.004 | |
| 2 | 55 | 8.46 | 3.36 | 28.57 | 0.013 |
| 75 | 16.03 | 3.46 | 29.43 | 0.020 | |
| 25 | 2.65 | 1.16 | 25.78 | 0.004 | |
| 3 | 55 | 8.49 | 3.37 | 27.19 | 0.010 |
| 75 | 15.84 | 3.48 | 29.43 | 0.012 | |
| 25 | 2.76 | 1.16 | 25.46 | 0.002 | |
| 4 | 55 | 9.54 | 3.37 | 27.13 | 0.003 |
| 75 | 19.44 | 3.48 | 29.43 | 0.007 | |
| 25 | 2.86 | 1.16 | 25.35 | 0.001 | |
| 5 | 55 | 9.88 | 3.37 | 27.02 | 0.002 |
| 75 | 20.12 | 3.49 | 29.33 | 0.022 | |
| 25 | 3.09 | 1.16 | 25.31 | 0.000 | |
| 6 | 55 | 10.63 | 3.36 | 27.01 | 0.003 |
| 75 | 21.57 | 3.48 | 29.09 | 0.026 | |
| 7 | 25 | 3.15 | 1.17 | 24.00 | 0.000 |
| 55 | 10.75 | 3.42 | 25.91 | 0.005 | |
| 75 | 21.76 | 3.49 | 27.49 | 0.039 | |
| 8 | 25 | 3.20 | 1.17 | 22.81 | 0.001 |
| 55 | 10.90 | 3.45 | 24.50 | 0.010 | |
| 75 | 22.03 | 3.49 | 25.88 | 0.063 | |
| 9 | 25 | 3.35 | 1.17 | 22.37 | 0.006 |
| 55 | 11.41 | 3.44 | 24.03 | 0.036 | |
| 75 | 23.03 | 3.54 | 25.36 | 0.119 |
| Cycle | Temperature (°C) | ± 0.02 × 10−2 [J·(mol·K)−1] | ± 0.01 [kJ·mol−1] | ± 0.01 [J·mol−1] |
|---|---|---|---|---|
| 25 | 6.04 | |||
| 1 | 55 | 6.03 | 29.08 | 9.58 |
| 75 | 10.25 | |||
| 2 | 25 | 6.05 | 29.13 | 6.04 |
| 55 | 9.58 | |||
| 75 | 9.90 | |||
| 3 | 25 | 6.07 | 29.18 | 6.03 |
| 55 | 9.57 | |||
| 75 | 9.68 | |||
| 4 | 25 | 6.08 | 29.23 | 6.02 |
| 55 | 9.56 | |||
| 75 | 9.67 | |||
| 25 | 6.14 | 29.32 | 6.02 | |
| 5 | 55 | 9.56 | ||
| 75 | 9.66 | |||
| 25 | 6.22 | 29.36 | 6.02 | |
| 6 | 55 | 9.55 | ||
| 75 | 9.63 | |||
| 25 | 6.30 | 29.43 | 6.02 | |
| 7 | 55 | 9.54 | ||
| 75 | 9.60 | |||
| 25 | 6.35 | 29.66 | 6.02 | |
| 8 | 55 | 9.53 | ||
| 75 | 9.60 | |||
| 25 | 6.65 | 29.87 | 6.01 | |
| 9 | 55 | 9.28 | ||
| 75 | 9.58 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina, O.E.; Gallego, J.; Restrepo, L.G.; Cortés, F.B.; Franco, C.A. Influence of the Ce4+/Ce3+ Redox-Couple on the Cyclic Regeneration for Adsorptive and Catalytic Performance of NiO-PdO/CeO2±δ Nanoparticles for n-C7 Asphaltene Steam Gasification. Nanomaterials 2019, 9, 734. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9050734
Medina OE, Gallego J, Restrepo LG, Cortés FB, Franco CA. Influence of the Ce4+/Ce3+ Redox-Couple on the Cyclic Regeneration for Adsorptive and Catalytic Performance of NiO-PdO/CeO2±δ Nanoparticles for n-C7 Asphaltene Steam Gasification. Nanomaterials. 2019; 9(5):734. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9050734
Chicago/Turabian StyleMedina, Oscar E., Jaime Gallego, Laura G. Restrepo, Farid B. Cortés, and Camilo A. Franco. 2019. "Influence of the Ce4+/Ce3+ Redox-Couple on the Cyclic Regeneration for Adsorptive and Catalytic Performance of NiO-PdO/CeO2±δ Nanoparticles for n-C7 Asphaltene Steam Gasification" Nanomaterials 9, no. 5: 734. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9050734