Morphology–Dependent Electrochemical Sensing Properties of Iron Oxide–Graphene Oxide Nanohybrids for Dopamine and Uric Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicas and Solutions
2.2. Apparatus
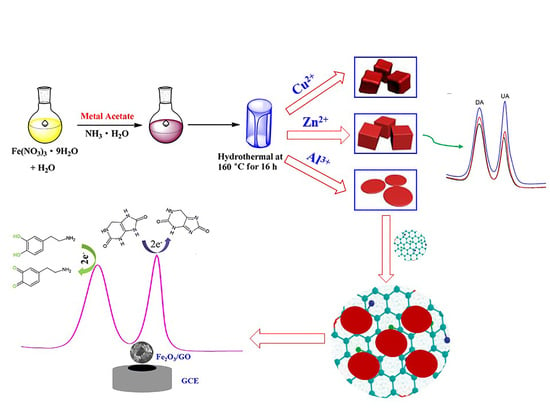
2.3. Synthesis of Discal, Thorhombic, and Cubic α-Fe2O3 NPs
2.4. Preparation of Fe2O3/GO Composite
2.5. Fabrication of Fe2O3/GO/GCE
2.6. Electrochemical Measurements
3. Results and Discussions
3.1. Morphology–Dependent Electrochemical Sensing of α-Fe2O3 NPs
3.2. Electrochemical Performance of d-Fe2O3/GO/GCE
3.3. Electrochemical Behaviors of DA and UA on the d-Fe2O3/GO/GCE
3.4. Optimation of Voltammetric Parameters
3.4.1. Influence of pH
3.4.2. Influence of Accumulation Parameters
3.5. Reaction Mechanism of DA and UA
3.6. Individual and Simultaneous Detection of DA and UA
3.7. Practical Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, Q.; Liu, J.; Liang, J.; Liu, X.; Li, W.; Liu, Z.; Ding, Z.; Tuo, D. Towards improvements for penetrating the blood–brain barrier—recent progress from a material and pharmaceutical perspective. Cells 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Dalley, J.W.; Roiser, J.P. Dopamine, serotonin and impulsivity. Neuroscience 2012, 215, 42–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlsson, A. Does dopamine play a role in schizophrenia? Psychol. Med. 1977, 7, 583–597. [Google Scholar] [CrossRef]
- Zhang, M.; Liao, C.; Yao, Y.; Liu, Z.; Gong, F.; Yan, F. High-Performance Dopamine Sensors Based on Whole-Graphene Solution-Gated Transistors. Adv. Funct. Mater. 2014, 24, 978–985. [Google Scholar] [CrossRef]
- Wightman, R.M.; May, L.J.; Michael, A.C. Detection of Dopamine Dynamics in the Brain. Anal. Chem. 1988, 60, 769A–793A. [Google Scholar] [CrossRef] [PubMed]
- Jindal, K.; Tomar, M.; Gupta, V. Nitrogen-doped zinc oxide thin films biosensor for determination of uric acid. Analyst 2013, 138, 4353–4362. [Google Scholar] [CrossRef]
- Aparna, T.K.; Sivasubramanian, R.; Dar, M.A. One-pot synthesis of Au-Cu2O/rGO nanocomposite based electrochemical sensor for selective and simultaneous detection of dopamine and uric acid. J. Alloys Compd. 2018, 741, 1130–1141. [Google Scholar] [CrossRef]
- Sharaf El Din, U.A.A.; Salem, M.M.; Abdulazim, D.O. Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: A review. J. Adv. Res. 2017, 8, 537–548. [Google Scholar] [CrossRef]
- Xiang, L.W.; Li, J.; Lin, J.M.; Li, H.F. Determination of gouty arthritis’ biomarkers in human urine using reversed-phase high-performance liquid chromatography. J. Pharm. Anal. 2014, 4, 153–158. [Google Scholar] [CrossRef]
- Lin, L.; Qiu, P.; Yang, L.; Cao, X.; Jin, L. Determination of dopamine in rat striatum by microdialysis and high-performance liquid chromatography with electrochemical detection on a functionalized multi-wall carbon nanotube electrode. Anal. Bioanal. Chem. 2006, 384, 1308–1313. [Google Scholar] [CrossRef]
- Zhao, D.; Song, H.; Hao, L.; Liu, X.; Zhang, L.; Lv, Y. Luminescent ZnO quantum dots for sensitive and selective detection of dopamine. Talanta 2013, 107, 133–139. [Google Scholar] [CrossRef]
- Moghadam, M.R.; Dadfarnia, S.; Shabani, A.M.H.; Shahbazikhah, P. Chemometric-assisted kinetic–spectrophotometric method for simultaneous determination of ascorbic acid, uric acid, and dopamine. Anal. Biochem. 2011, 410, 289–295. [Google Scholar] [CrossRef]
- Huang, C.; Chen, X.; Lu, Y.; Yang, H.; Yang, W. Electrogenerated chemiluminescence behavior of peptide nanovesicle and its application in sensing dopamine. Biosens. Bioelectron. 2015, 63, 478–482. [Google Scholar] [CrossRef]
- Kumbhat, S.; Shankaran, D.R.; Kim, S.J.; Gobi, K.V.; Joshi, V.; Miura, N. Surface plasmon resonance biosensor for dopamine using D3 dopamine receptor as a biorecognition molecule. Biosens. Bioelectron. 2007, 23, 421–427. [Google Scholar] [CrossRef]
- Liu, S.; Yan, J.; He, G.; Zhong, D.; Chen, J.; Shi, L.; Zhou, X.; Jiang, H. Layer-by-layer assembled multilayer films of reduced graphene oxide/gold nanoparticles for the electrochemical detection of dopamine. J. Electroanal. Chem. 2012, 672, 40–44. [Google Scholar] [CrossRef]
- Qi, S.; Zhao, B.; Tang, H.; Jiang, X. Determination of ascorbic acid, dopamine, and uric acid by a novel electrochemical sensor based on pristine graphene. Electrochim. Acta 2015, 161, 395–402. [Google Scholar] [CrossRef]
- Lian, Q.; He, Z.; He, Q.; Luo, A.; Yan, K.; Zhang, D.; Lu, X.; Zhou, X. Simultaneous determination of ascorbic acid, dopamine and uric acid based on tryptophan functionalized graphene. Anal. Chim. Acta 2014, 823, 32–39. [Google Scholar] [CrossRef]
- Hou, J.; Xu, C.; Zhao, D.; Zhou, J. Facile fabrication of hierarchical nanoporous AuAg alloy and its highly sensitive detection towards dopamine and uric acid. Sens. Actuators B Chem. 2016, 225, 241–248. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Xia, Y.; Li, G.; Deng, P.; Chen, D. Novel Electrochemical Sensors Based on Cuprous Oxide-Electrochemically Reduced Graphene Oxide Nanocomposites Modified Electrode toward Sensitive Detection of Sunset Yellow. Molecules 2018, 23, 2130. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Chen, D.; Deng, P.; Liang, J. A promising sensing platform toward dopamine using MnO2 nanowires/electro-reduced graphene oxide composites. Electrochim. Acta 2019, 296, 683–692. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Manganese dioxide Nanorods/electrochemically reduced graphene oxide nanocomposites modified electrodes for cost-effective and ultrasensitive detection of Amaranth. Colloids Surf. B 2018, 172, 565–572. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J.; Chen, D. Sensitive and Selective Detection of Tartrazine Based on TiO2-Electrochemically Reduced Graphene Oxide Composite-Modified Electrodes. Sensors 2018, 18, 1911. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Chen, D.; Deng, P.; Liang, J. Fabrication of Amine-Modified Magnetite-Electrochemically Reduced Graphene Oxide Nanocomposite Modified Glassy Carbon Electrode for Sensitive Dopamine Determination. Nanomaterials 2018, 8, 194. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Preparation of Cu2O-Reduced Graphene Nanocomposite Modified Electrodes towards Ultrasensitive Dopamine Detection. Sensors 2018, 18, 199. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.X.; Zheng, J.-N.; Wang, A.J.; Wu, L.J.; Chen, J.R.; Feng, J.J. Facile synthesis of porous bimetallic alloyed PdAg nanoflowers supported on reduced graphene oxide for simultaneous detection of ascorbic acid, dopamine, and uric acid. Analyst 2015, 140, 3183–3192. [Google Scholar] [CrossRef]
- Zhao, L.; Li, H.; Gao, S.; Li, M.; Xu, S.; Li, C.; Guo, W.; Qu, C.; Yang, B. MgO nanobelt-modified graphene-tantalum wire electrode for the simultaneous determination of ascorbic acid, dopamine and uric acid. Electrochim. Acta 2015, 168, 191–198. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, W.; Zhang, J.; Li, Y.; Tang, W.; Xu, Q. NiCo-embedded in hierarchically structured N-doped carbon nanoplates for the efficient electrochemical determination of ascorbic acid, dopamine, and uric acid. RSC Adv. 2015, 5, 65532–65539. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Lin, X.; Wan, H.; Zhang, S. Electrocatalytic Oxidation and Determination of Dopamine in the Presence of Ascorbic Acid and Uric Acid at a Poly (4-(2-Pyridylazo)-Resorcinol) Modified Glassy Carbon Electrode. Electroanalysis 2007, 19, 612–615. [Google Scholar] [CrossRef]
- Li, Y.; Lin, X. Simultaneous electroanalysis of dopamine, ascorbic acid and uric acid by poly (vinyl alcohol) covalently modified glassy carbon electrode. Sens. Actuators B 2006, 115, 134–139. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Zheng, X.-Q.; Xu, J.Y.; Bao, W.J.; Wang, F.B.; Xia, X.H. Electrochemical sensor based on nitrogen doped graphene: Simultaneous determination of ascorbic acid, dopamine and uric acid. Biosens. Bioelectron. 2012, 34, 125–131. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Hou, H.; You, T. Simultaneous determination of dopamine, ascorbic acid and uric acid with electrospun carbon nanofibers modified electrode. Electrochem. Commun. 2008, 10, 1431–1434. [Google Scholar] [CrossRef]
- Cui, R.; Wang, X.; Zhang, G.; Wang, C. Simultaneous determination of dopamine, ascorbic acid, and uric acid using helical carbon nanotubes modified electrode. Sens. Actuators B Chem. 2012, 161, 1139–1143. [Google Scholar] [CrossRef]
- Harraz, F.A.; Ismail, A.A.; Al-Sayari, S.A.; Al-Hajry, A.; Al-Assiri, M.S. Highly sensitive amperometric hydrazine sensor based on novel α-Fe2O3/crosslinked polyaniline nanocomposite modified glassy carbon electrode. Sens. Actuators B Chem. 2016, 234, 573–582. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahn, M.A.; Hahn, Y. A Highly Sensitive Nonenzymatic Sensor Based on Fe2O3 Nanoparticle Coated ZnO Nanorods for Electrochemical Detection of Nitrite. Adv. Mater. Interfaces 2017, 4, 1700491. [Google Scholar] [CrossRef]
- Larsen, G.K.; Farr, W.; Murph, S.E.H. Multifunctional Fe2O3–Au Nanoparticles with Different Shapes: Enhanced Catalysis, Photothermal Effects, and Magnetic Recyclability. J. Phys. Chem. C 2016, 120, 15162–15172. [Google Scholar] [CrossRef]
- Quan, H.; Cheng, B.; Xiao, Y.; Lei, S. One-pot synthesis of α-Fe2O3 nanoplates-reduced graphene oxide composites for supercapacitor application. Chem. Eng. J. 2016, 286, 165–173. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wang, Y.X.; Chou, S.L.; Li, H.J.; Liu, H.K.; Wang, J.Z. Rapid synthesis of α-Fe2O3/rGO nanocomposites by microwave autoclave as superior anodes for sodium-ion batteries. J. Power Sources 2015, 280, 107–113. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Yi, H.; Wei, H.; Guo, Z.; Wang, X. One-step preparation of single-crystalline Fe2O3 particles/graphene composite hydrogels as high performance anode materials for supercapacitors. Nano Energy 2014, 7, 86–96. [Google Scholar] [CrossRef]
- Cummings, C.Y.; Bonné, M.J.; Edler, K.J.; Helton, M.; Mckee, A.; Marken, F. Direct reversible voltammetry and electrocatalysis with surface-stabilised Fe2O3 redox states. Electrochem. Commun. 2008, 10, 1773–1776. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, R.; Xu, J.; Chen, J.; Yan, Y.; Narlikar, A.; Zhang, H. Synthesis of large arrays of aligned α-Fe2O3 nanowires. Chem. Phys. Lett. 2003, 379, 373–379. [Google Scholar] [CrossRef]
- Liu, L.; Kou, H.-Z.; Mo, W.; Liu, H.; Wang, Y. Surfactant-assisted synthesis of α-Fe2O3 nanotubes and nanorods with shape-dependent magnetic properties. J. Phys. Chem. B 2006, 110, 15218–15223. [Google Scholar] [CrossRef]
- Mou, X.; Zhang, B.; Li, Y.; Yao, L.; Wei, X.; Su, D.S.; Shen, W.J. Rod-shaped Fe2O3 as an efficient catalyst for the selective reduction of nitrogen oxide by ammonia. Angew. Chem. Int. Ed. 2012, 51, 2989–2993. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, H.; Su, C.; Zhang, P.; Bai, J. Controlled fabrication and characterization of microspherical FeCO3 and α-Fe2O3. J. Colloid Interface Sci. 2010, 351, 427–432. [Google Scholar] [CrossRef]
- Fu, X.; Bei, F.; Wang, X.; Yang, X.; Lu, L. Surface-enhanced Raman scattering of 4-mercaptopyridine on sub-monolayers of α-Fe2O3 nanocrystals (sphere, spindle, cube). J. Raman Spectrosc. 2009, 40, 1290–1295. [Google Scholar] [CrossRef]
- Yan, W.; Fan, H.; Zhai, Y.; Yang, C.; Ren, P.; Huang, L. Low temperature solution-based synthesis of porous flower-like α-Fe2O3 superstructures and their excellent gas-sensing properties. Sens. Actuators B 2011, 160, 1372–1379. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, B.; Ding, Z.; Zheng, H.; Huang, L.; Pan, J.; Wu, W.; Zhang, H. Tetragonal hematite single crystals as anode materials for high performance lithium ion batteries. J. Power Sources 2015, 286, 124–129. [Google Scholar] [CrossRef]
- Liu, J.; Yang, S.; Wu, W.; Tian, Q.; Cui, S.; Dai, Z.; Ren, F.; Xiao, X.; Jiang, C. 3D Flowerlike α-Fe2O3@TiO2 Core–Shell Nanostructures: General Synthesis and Enhanced Photocatalytic Performance. ACS Sustain. Chem. Eng. 2015, 3, 2975–2984. [Google Scholar] [CrossRef]
- Wu, W.; Yang, S.; Pan, J.; Sun, L.; Zhou, J.; Dai, Z.; Xiao, X.; Zhang, H.; Jiang, C. Metal ion-mediated synthesis and shape-dependent magnetic properties of single-crystalline α-Fe2O3 nanoparticles. CrystEngComm 2014, 16, 5566–5572. [Google Scholar] [CrossRef]
- Yin, C.Y.; Minakshi, M.; Ralph, D.E.; Jiang, Z.T.; Xie, Z.; Guo, H.J. Hydrothermal synthesis of cubic α-Fe2O3 microparticles using glycine: Surface characterization, reaction mechanism and electrochemical activity. J. Alloys Compd. 2011, 509, 9821–9825. [Google Scholar] [CrossRef]
- Chen, A.; Liang, X.; Zhang, X.; Yang, Z.; Yang, S. Improving Surface Adsorption via Shape Control of Hematite α-Fe2O3 Nanoparticle for Sensitive Dopamine Sensors. ACS Appl. Mater. Interfaces 2016, 8, 33765–33774. [Google Scholar] [CrossRef]
- Mitra, S.; Das, S.; Mandal, K.; Chaudhuri, S. Synthesis of a α-Fe2O3 nanocrystal in its different morphological attributes: growth mechanism, optical and magnetic properties. Nanotechnology 2007, 18, 275608. [Google Scholar] [CrossRef]
- Jagadeesan, D.; Mansoori, U.; Mandal, P.; Sundaresan, A.; Eswaramoorthy, M. Hollow Spheres to Nanocups: Tuning the Morphology and Magnetic Properties of Single-Crystalline α-Fe2O3 Nanostructures. Angew. Chem. Int. Ed. 2008, 47, 7685–7688. [Google Scholar] [CrossRef]
- Liu, X.; Chen, T.; Chu, H.; Niu, L.; Sun, Z.; Pan, L.; Sun, C.Q. Fe2O3-reduced graphene oxide composites synthesized via microwave-assisted method for sodium ion batteries. Electrochim. Acta 2015, 166, 12–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, W.; Zuo, L.; Zhang, L.; Huang, Y.; Lu, H.; Fan, W.; Liu, T. In situ growth of Fe2O3 nanoparticles on highly porous graphene/polyimide-based carbon aerogel nanocomposites for Effectively selective detection of dopamine. Adv. Mater. Interfaces 2016, 3, 1600137. [Google Scholar] [CrossRef]
- Yasmin, S.; Ahmed, M.S.; Jeon, S. Determination of dopamine by dual doped graphene-Fe2O3 in Presence of Ascorbic Acid. J. Electrochem. Soc. 2015, 162, B363–B369. [Google Scholar] [CrossRef]
- Gan, T.; Shi, Z.; Deng, Y.; Sun, J.; Wang, H. Morphology–dependent electrochemical sensing properties of manganese dioxide–graphene oxide hybrid for guaiacol and vanillin. Electrochim. Acta 2014, 147, 157–166. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Duan, Y.Y. Towards conductive-gel-free electrodes: Understanding the wet electrode, semi-dry electrode and dry electrode-skin interface impedance using electrochemical impedance spectroscopy fitting. Sens. Actuators B Chem. 2018, 277, 250–260. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Duan, Y.Y. Towards gel-free electrodes: A systematic study of electrode-skin impedance. Sens. Actuators B Chem. 2017, 241, 1244–1255. [Google Scholar] [CrossRef]
- Li, G.; Zhang, D.; Wang, S.; Duan, Y.Y. Novel passive ceramic based semi-dry electrodes for recording electroencephalography signals from the hairy scalp. Sens. Actuators B Chem. 2016, 237, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yang, B.; Zhong, J.; Yan, B.; Zhang, K.; Zhai, C.; Shiraishi, Y.; Du, Y.; Yang, P. Dopamine and uric acid electrochemical sensor based on a glassy carbon electrode modified with cubic Pd and reduced graphene oxide nanocomposite. J. Colloid Interface Sci. 2017, 497, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.Q.; Zhang, Q.L.; Zheng, J.N.; Lv, Z.Y.; Wei, J.; Wang, A.J.; Feng, J.J. Simultaneous determination of dopamine and uric acid in the presence of ascorbic acid using Pt nanoparticles supported on reduced graphene oxide. Electrochim. Acta 2014, 115, 109–115. [Google Scholar] [CrossRef]
- Kogularasu, S.; Akilarasan, M.; Chen, S.-M.; Chen, T.W.; Lou, B.S. Urea-based morphological engineering of ZnO; for the biosensing enhancement towards dopamine and uric acid in food and biological samples. Mater. Chem. Phys. 2019, 227, 5–11. [Google Scholar] [CrossRef]
- Teo, P.S.; Alagarsamy, P.; Huang, N.M.; Lim, H.N.; Yusran, S. Simultaneous electrochemical detection of dopamine and ascorbic acid using an iron oxide/reduced graphene oxide modified glassy carbon electrode. Sensors 2014, 14, 15227–15243. [Google Scholar]
- Zhang, K.; Chen, X.; Li, Z.; Wang, Y.; Sun, S.; Wang, L.N.; Guo, T.; Zhang, D.; Xue, Z.; Zhou, X. Au-Pt bimetallic nanoparticles decorated on sulfonated nitrogen sulfur co-doped graphene for simultaneous determination of dopamine and uric acid. Talanta 2018, 178, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; She, P.; Gong, J.; Wu, W.; Xu, S.; Li, J.; Zhao, K.; Deng, A. A novel sensor based on electrodeposited Au–Pt bimetallic nano-clusters decorated on graphene oxide (GO)–electrochemically reduced GO for sensitive detection of dopamine and uric acid. Sens. Actuators B Chem. 2015, 221, 1542–1553. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, X.; Zheng, J. A facile one-step synthesis of Fe 2 O 3 /nitrogen-doped reduced graphene oxide nanocomposite for enhanced electrochemical determination of dopamine. J. Alloys Compd. 2017, 709, 581–587. [Google Scholar] [CrossRef]
- Ghanbari, K.; Moloudi, M. Flower-like ZnO decorated polyaniline/reduced graphene oxide nanocomposites for simultaneous determination of dopamine and uric acid. Anal. Biochem. 2016, 512, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.P.; Feng, J.J.; Wu, L.; Chen, J.R.; Shen, L.; Xie, Y.; Wang, A.J. A glassy carbon electrode modified with porous Cu2O nanospheres on reduced graphene oxide support for simultaneous sensing of uric acid and dopamine with high selectivity over ascorbic acid. Microchim. Acta 2016, 183, 2039–2046. [Google Scholar] [CrossRef]
- Asif, M.; Aziz, A.; Wang, H.; Wang, Z.; Wang, W.; Ajmal, M.; Xiao, F.; Chen, X.; Liu, H. Superlattice stacking by hybridizing layered double hydroxide nanosheets with layers of reduced graphene oxide for electrochemical simultaneous determination of dopamine, uric acid and ascorbic acid. Microchim. Acta 2019, 186, 61. [Google Scholar] [CrossRef]
- Sumathi, C.; Venkateswara Raju, C.; Muthukumaran, P.; Wilson, J.; Ravi, G. Au–Pd bimetallic nanoparticles anchored on α-Fe2O3 nonenzymatic hybrid nanoelectrocatalyst for simultaneous electrochemical detection of dopamine and uric acid in the presence of ascorbic acid. J. Mater. Chem. B 2016, 4, 2561–2569. [Google Scholar] [CrossRef]













| Electrodes | Methods | Detection Range (μM) | LOD (μM) | Ref. | ||
|---|---|---|---|---|---|---|
| DA | UA | DA | UA | |||
| Au/Cu2O/rGO/GCE | DPV | 10–90 | 100–900 | 3.9 | 6.5 | [7] |
| NSG–Fe2O3/GCE | DPV | 0.3–210 | 0.035 | [55] | ||
| Pd/RGO/GCE | DPV | 0.45–71 | 6–469.5 | 0.18 | 1.6 | [60] |
| Pt/RGO/GCE | DPV | 10–170 | 10–130 | 0.25 | 0.45 | [61] |
| ZnO/SPCE | DPV | 0.1–374 | 0.1–169 | 0.004 | 0.00849 | [62] |
| Fe3O4/rGO/GCE | DPV | 0.5–100 | 0.12 | [63] | ||
| AuPtNPs/S-NS-GR/GCE | DPV | 0.01–400 | 1–1000 | 0.006 | 0.0038 | [64] |
| Au–Pt/GO–ERGO | DPV | 0.0682–49,800 | 0.125–82,800 | 0.0207 | 0.0407 | [65] |
| Fe2O3/NrGO/GCE | Amperometry | 0.5–340 | 0.49 | [66] | ||
| ZnO/PANI/rGO/GCE | DPV | 0.1–90 | 0.5–90 | 0.017 | 0.12 | [67] |
| pCu2O NS-rGO/GCE | DPV | 0.05–109 | 1–138 | 0.015 | 0.112 | [68] |
| Zn-NiAl LDH/rGO/GCE | DPV | 0.0001–1 | 0.0011–0.95 | 0.0001 | 0.0009 | [69] |
| α-Fe2O3@Au-Pd/GCE | SWV | 0.1–1000 | 1–1000 | 0.0000138 | 0.97 | [70] |
| d-Fe2O3/GO/GCE | DPV | 0.02–10; 10–100 | 0.02–10; 10–100 | 0.0032 | 0.0025 | This work |
| Samples a | Detected (μM) | Added (μM) | Found (μM) | RSD (%) | Recovery (%) | |
|---|---|---|---|---|---|---|
| Serum | DA | ND b | 20 | 18.82 | 3.75 | 94.1 |
| 40 | 38.26 | 2.86 | 95.7 | |||
| UA | 20.6 | 20 | 41.76 | 2.75 | 105.8 | |
| 40 | 63.86 | 2.21 | 108.2 | |||
| Urine | DA | ND b | 20 | 21.06 | 4.98 | 105.3 |
| 40 | 42.56 | 3.67 | 106.4 | |||
| UA | 35.24 | 20 | 54.27 | 2.62 | 95.2 | |
| 40 | 74.52 | 1.39 | 98.2 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Z.; Ye, Y.; Wan, X.; Liu, J.; Yang, S.; Xia, Y.; Li, G.; He, Q. Morphology–Dependent Electrochemical Sensing Properties of Iron Oxide–Graphene Oxide Nanohybrids for Dopamine and Uric Acid. Nanomaterials 2019, 9, 835. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9060835
Cai Z, Ye Y, Wan X, Liu J, Yang S, Xia Y, Li G, He Q. Morphology–Dependent Electrochemical Sensing Properties of Iron Oxide–Graphene Oxide Nanohybrids for Dopamine and Uric Acid. Nanomaterials. 2019; 9(6):835. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9060835
Chicago/Turabian StyleCai, Zhaotian, Yabing Ye, Xuan Wan, Jun Liu, Shihui Yang, Yonghui Xia, Guangli Li, and Quanguo He. 2019. "Morphology–Dependent Electrochemical Sensing Properties of Iron Oxide–Graphene Oxide Nanohybrids for Dopamine and Uric Acid" Nanomaterials 9, no. 6: 835. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9060835