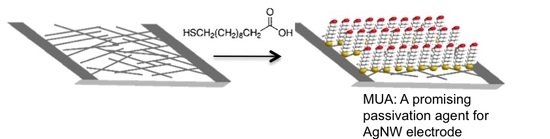
Increasing Silver Nanowire Network Stability through Small Molecule Passivation
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sannicolo, T.; Lagrange, M.; Cabos, A.; Celle, C.; Simonato, J.P.; Bellet, D. Metallic Nanowire-Based Transparent Electrodes for Next Generation Flexible Devices: A Review. Small 2016, 12, 6052–6075. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Rathmell, A.R.; Chen, Z.; Stewart, I.E.; Wiley, B.J. Metal nanowire networks: The next generation of transparent conductors. Adv. Mater. 2014, 26, 6670–6687. [Google Scholar] [CrossRef] [PubMed]
- Bellet, D.; Lagrange, M.; Sannicolo, T.; Aghazadehchors, S.; Nguyen, V.H.; Langley, D.; Munoz-Rojas, D.; Jimenez, C.; Bréchet, Y.; Nguyen, N.D. Transparent Electrodes Based on Silver Nanowire Networks: From Physical Considerations towards Device Integration. Materials 2017, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y. Silver nanowires-unique templates for functional nanostructures. Nanoscale 2010, 9, 1626–1642. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Kim, H.S.; Lee, J.; Peumans, P.; Cui, Y. Scalable coating and properties of transparent, flexible, silver nanowire electrodes. ACS Nano 2010, 4, 2955–2963. [Google Scholar] [CrossRef] [PubMed]
- Madaria, A.R.; Kumar, A.; Zhou, C. Large scale, highly conductive and patterned transparent films of silver nanowires on arbitrary substrates and their application in touch screens. Nanotechnology 2011, 22, 245201–245208. [Google Scholar] [CrossRef]
- Pasquarelli, R.M.; Ginley, D.S.; O’Hayre, R. Solution processing of transparent conductors: From flask to film. Chem. Soc. Rev. 2011, 40, 5406–5441. [Google Scholar] [CrossRef]
- Zhang, R.; Engholm, M. Recent progress on the fabrication and properties of silver nanowire-base transparent electrodes. Nanomaterials 2018, 8, 628. [Google Scholar] [CrossRef]
- Elechiguerra, J.L.; Larios-Lopez, L.; Lui, C.; Garcia-Gutierrez, D.; Camacho-Bragada, A.; Yacaman, M.J. Corrosion at the nanoscale: The case of silver nanowires and nanoparticles. Chem. Mater. 2005, 17, 6042–6052. [Google Scholar] [CrossRef]
- Jiu, J.; Wang, J.; Sugahara, T.; Nagao, S.; Nogi, M.; Koga, H.; Suganuma, K.; Hara, M.; Nakazawa, E.; Uchida, H. The Effect of Light and Humidity on the Stability of Silver Nanowire Transparent Electrodes. RSC Adv. 2015, 5, 27657–27664. [Google Scholar] [CrossRef]
- Mayousse, C.; Celle, C.; Fraczkiewicz, A.; Simonato, J.P. Stability of silver nanowire based electrodes under environmental and electrical stresses. Nanoscale 2015, 7, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Deignan, G.; Goldthorpe, I.A. The dependance of silver nanowire stability on network compositions and processing parameters. RSC Adv. 2017, 7, 35590–35597. [Google Scholar] [CrossRef]
- Franey, J.P.; Kammlott, J.P.; Graedel, T.E. The corrosion of silver by atmospheric sulfurous gases. Corros. Sci. 1985, 25, 33–143. [Google Scholar] [CrossRef]
- Keast, V.J.; Myles, T.A.; Shahcheraghi, N.; Cortie, M.B. Corrosion processes of triangular silver nanoparticles compared to bulk silver. J. Nanopart. Res. 2016, 18, 45–56. [Google Scholar] [CrossRef]
- Wang, X.; Santshi, C.; Martin, O. Strong improvement of long-term chemical and thermal stability of plasmonic silver nanoantennas and films. Small 2017, 13, 1700044–1700049. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Buchman, J.T.; Rodriguez, R.S.; Ring, H.T.; He, J.; Bantz, K.C.; Haynes, C.L. Stabilization of silver and gold nanoparticles: Preservation and improvement of plasmonic functionalities. Chem. Rev. 2019, 119, 664–699. [Google Scholar] [CrossRef]
- Chen, S.; Goode, A.E.; Sweeney, S.; Theodorou, I.G.; Thorley, A.J.; Ruenraroengsak, P.; Chang, Y.; Gow, A.; Schwander, S.; Skepper, J.; et al. Sulfidation of silver nanowires inside human alveolar epithelial cells: A potential detoxification mechanism. Nanoscale 2013, 5, 9839–9847. [Google Scholar] [CrossRef]
- Moon, I.K.; Kim, J.I.; Lee, H.; Hur, K.; Kim, W.C.; Lee, H. 2D Graphen oxide nanosheetes as an adhesive over-coating layer for flexible transparent conductive electrodes. Sci. Rep. 2013, 3, 1112. [Google Scholar] [CrossRef]
- Song, T.B.; Rim, Y.S.; Liu, F.; Bob, B.; Ye, S.; Hsieh, T.; Yang, Y. Highly Robust Silver Nanowire Network for Transparent Electrode. ACS Appl. Mat. Interfaces 2015, 7, 24601–24607. [Google Scholar] [CrossRef]
- Deng, B.; Hsu, P.-C.; Chen, G.; Chandrashekar, B.N.; Liao, L.; Ayitimuda, Z.; Wu, J.; Guo, Y.; Zhou, Y.; Aisijiang, M.; et al. Roll-to-Roll Encapsulation of Metal Nanowires between Graphene and Plastic Substrate for High-Performance Flexible Transparent Electrodes. Nano Lett. 2015, 15, 4206–4213. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, S.; Araki, T.; Jiu, J.; Sugahara, T.; Wang, J.; Vanfleteren, J.; Sekitani, T.; Suganuma, K. Facile Fabrication of Stretchable Ag Nanowire/Polyurethane Electrodes Using High Intensity Pulsed Light. Nano Res. 2016, 9, 401–414. [Google Scholar] [CrossRef]
- Yoo, Y.Z.; Na, J.-Y.; Choi, Y.S.; Lim, Y.J.; Kim, J.-H.; Kim, Y.B.; Kim, S.; Seong, T. An Optically Flat Conductive Outcoupler Using Core/Shell Ag/ZnO Nanochurros. Small 2018, 14, 1800056. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Hwang, B.; Kim, J. Ambient-stable and durable conductive Ag-Nanowire-network, 2D fims decorated with a Ti layer. Nanomaterials 2018, 8, 321. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, B.; He, Z.; Zhao, X.; Wang, H.; Yang, S.; Wu, H.; Cao, Y. High-efficiency ITO-free polymer solar cells using highly conductive PEDOT: PSS surfactant bilayer transparent anodes. Energy Environ. Sci. 2013, 6, 1956–1964. [Google Scholar] [CrossRef]
- Chen, S.; Song, L.; Tao, Z.; Shao, X.; Huang, Y.; Cui, Q.; Guo, X. Neutral-pH PEDOT: PSS as over-coating layer for stable silver nanowire flexible transparent conductive films. Org. Electron. 2014, 15, 3654–3659. [Google Scholar] [CrossRef]
- Ahn, Y.; Jeong, Y.; Lee, Y. Improved thermal oxidation stability of solution-processable silver nanowire transparent electrode by reduced graphene oxide. ACS Appl. Mater. Interfaces 2012, 4, 6410–6414. [Google Scholar] [CrossRef]
- Lee, D.; Lee, H.; Ahn, Y.; Lee, Y. High-performance flexible transparent conductive film based on graphene/AgNW/graphene sandwich structure. Carbon 2015, 81, 439–446. [Google Scholar] [CrossRef]
- Idier, J.; Neri, W.; Labrugere, C.; Ly, I.; Poulin, P.; Backov, R. Modified silver nanowire transparent electrodes with exceptional stability against oxidation. Nanotechnology 2016, 27, 105705–105712. [Google Scholar] [CrossRef]
- Liu, G.S.; Qiu, J.S.; Xu, D.H.; Zhou, X.; Zhong, D.; Shieh, H.P.D.; Yang, B.R. Fabrication of Embedded Silver Nanowires on Arbitrary Substrates with Enhanced Stability via Chemisorbed Alkanethiolate. ACS Appl. Mater. Interfaces 2017, 9, 15130–15138. [Google Scholar] [CrossRef]
- Liu, G.; Xu, Y.; Kong, Y.; Wang, L.; Wang, J.; Xie, X.; Luo, Y.; Yang, B. Comprehensive stability improvement of silver nanowire networks via self-assembled mercapto inhibitors. ACS Appl. Mater. Interfaces 2018, 10, 37699–37708. [Google Scholar] [CrossRef]
- Gooding, J.J.; Ciampi, S. The molecular level modification of surfaces: From self-assembled monolayers to complex molecular assemblies. Chem. Soc. Rev. 2011, 40, 2704–2718. [Google Scholar] [CrossRef] [PubMed]
- Le Beulze, A.; Duguet, E.; Mornet, S.; Majimel, J.; Tréguer-Delapierre, M.; Ravaine, S.; Florea, I.; Ersen, O. New Insights into the Side-Face Structure, Growth Aspects, and Reactivity of Agn Nanoprisms. Langmuir 2014, 30, 1424–1434. [Google Scholar] [CrossRef]
- Fajín, J.L.; Teixeira, F.; Gomes, J.R.; Cordeiro, M.N.D. Effect of van der Waals interactions in the DFT description of self-assembled monolayers of thiols on gold. In Proceedings of the 9th Congress on Electronic Structure: Principles and Applications, Badajoz, Spain, 2–4 July 2014; Springer: Berlin/Heidelberg, Germany, 2016; pp. 127–139. [Google Scholar]
- Pan, X.-F.; Gao, H.-L.; Su, Y.; Wu, Y.-D.; Wang, X.-Y.; Xue, J.-Z.; He, T.; Lu, Y.; Liu, J.; Yu, S. Strong and stiff Ag nanowire-chitosan composite films reinforced by Ag-S covalent bonds. Nano Res. 2018, 11, 410–419. [Google Scholar] [CrossRef]
- Plissonneau, M.; Madeira, A.; Talaga, D.; Bonhommeau, S.; Servant, L.; Vallée, R.; Labrugère, C.; Goldthorpe, I.; Pautrot-d’Alençon, L.; Le Mercier, T.; et al. Efficient Passivation of Ag Nanowires with 11-Mercaptoundecanoic Acid Probed Using In-Situ Total-Internal-Reflection Surface-Enhanced Raman Scattering Spectroscopy. ChemNanoMat 2019, 5, 1–7. [Google Scholar] [CrossRef]
- Plissonneau, M.; Pautrot-D’Alençon, L.; Le Mercier, T.; Tréguer-Delapierre, M. Process for the Manufacture of Metal Nanowires. European Patent No 17306072.4-1103, 13 August 2017. [Google Scholar]
- Grillet, N.; Manchon, D.; Cottancin, E.; Bertorelle, F.; Bonnet, C.; Broyer, M.; Lermé, J.; Pellarin, M. Photo-oxidation of individual silver nanoparticles: A real-time tracking of optical and morphological changes. J. Phys. Chem. C 2013, 117, 2274–2282. [Google Scholar] [CrossRef]



| Unpassivated | MUA-Passivated | |
|---|---|---|
| Rs (Ω/Sq) | 17.4 ± 2.0 | 17.0 ± 2.0 |
| T | 70 ± 1% | 71 ± 2% |
| Storage conditions | Daylight | Dark | ||
|---|---|---|---|---|
| Nanowire surface | MUA-passivated | Unpassivated | MUA-Passivated | |
| After 4 days | +0.8% | +0.8% | +0.8% | −0.1% |
| After 3 months | −2.3% | −1.1% | −1.1% | −2.1% |
| After 4 months | −4.1% | −1.8% | −1.8% | −3.0% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madeira, A.; Plissonneau, M.; Servant, L.; Goldthorpe, I.A.; Tréguer-Delapierre, M. Increasing Silver Nanowire Network Stability through Small Molecule Passivation. Nanomaterials 2019, 9, 899. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9060899
Madeira A, Plissonneau M, Servant L, Goldthorpe IA, Tréguer-Delapierre M. Increasing Silver Nanowire Network Stability through Small Molecule Passivation. Nanomaterials. 2019; 9(6):899. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9060899
Chicago/Turabian StyleMadeira, Alexandra, Marie Plissonneau, Laurent Servant, Irene A. Goldthorpe, and Mona Tréguer-Delapierre. 2019. "Increasing Silver Nanowire Network Stability through Small Molecule Passivation" Nanomaterials 9, no. 6: 899. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9060899