The Origins of Enhanced and Retarded Crystallization in Nanocomposite Polymers
Abstract
:1. Introduction
2. Methodology
3. Results and Discussion
3.1. The Effect of Particle Size
3.2. Effect of Nanoparticle Volume Fraction φ
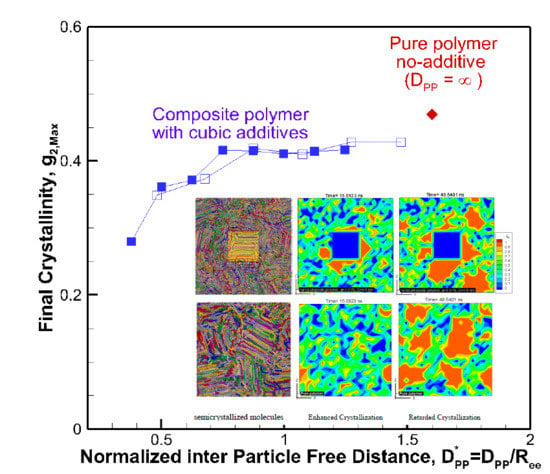
3.3. Combined Effects of Volume Fraction and Particle Size
3.4. Critical Volume Fraction and Particle Size
4. Correlation with Experimental Results
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Jeziorska, R.; Szadkowska, A.; Zielecka, M.; Wenda, M.; Kepska, B. Morphology and thermal properties of HDPE nanocomposites: Effect of spherical silica surface modification and compatibilizer. Polym. Degrad. Stab. 2017, 145, 70–78. [Google Scholar] [CrossRef]
- Khan, J.; Harton, S.E.; Akcora, P.; Benicewicz, B.C.; Kumar, S.K. Polymer crystallization in nanocomposites: Spatial reorganization of nanoparticles. Macromolecules 2009, 42, 5741–5744. [Google Scholar] [CrossRef]
- Zhao, D.; Gimenez-Pinto, V.; Jimenez, A.M.; Zhao, L.; Jestin, J.; Kumar, S.K.; Khani, M.M. Tunable multiscale nanoparticle ordering by polymer crystallization. ACS Cent. Sci. 2017, 3, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Shukla, D.; Kumar, V. Studies on effect of nano-talc filler on nucleation, crystal morphology and crystallization behaviour of semi-crystalline plastics. In Solid State Phenomena; Trans Tech Publications: Zurich, The Switzerland, 2008; Volume 136, pp. 161–174. [Google Scholar]
- Bosq, N.; Aht-Ong, D. Nonisothermal crystallization behaviour of poly (butylene succinate)/nay zeolite nanocomposites. Macromol. Res. 2008, 26, 13–21. [Google Scholar] [CrossRef]
- Reinsch, V.E.; Rebenfeld, L. Crystallization processes in poly (ethylene terephthalate) as modified by polymer additives and fiber reinforcement. J. Appl. Polym. Sci. 1994, 52, 649–662. [Google Scholar] [CrossRef]
- Weng, W.; Chen, G.; Wu, D. Crystallization kinetics and melting behaviors of nylon 6/foliated graphite nanocomposites. Polymer 2003, 44, 8119–8132. [Google Scholar] [CrossRef]
- Krikorian, V.; Pochan, D.J. Unusual crystallization behavior of organoclay reinforced poly (L-lactic acid) nanocomposites. Macromolecules 2004, 37, 6480–6491. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, S.; Kota, S.; Pan, Q.; Barsoum, M.W.; Li, C.Y. Structure and crystallization behavior of poly (ethylene oxide)/Ti3C2Tx MXene nanocomposites. Polymer 2016, 102, 119–126. [Google Scholar] [CrossRef]
- Li, L.; Li, C.Y.; Ni, C.; Rong, L.; Hsiao, B. Structure and crystallization behavior of Nylon 66/multi-walled carbon nanotube nanocomposites at low carbon nanotube contents. Polymer 2007, 48, 3452–3460. [Google Scholar] [CrossRef]
- Papananou, H.; Perivolari, E.; Chrissopoulou, K.; Anastasiadis, S.H. Tuning polymer crystallinity via the appropriate selection of inorganic nanoadditives. Polymer 2008, 157, 111–121. [Google Scholar] [CrossRef]
- Yamamoto, T. Computer modeling of polymer crystallization–Toward computer-assisted materials’ design. Polymer 2009, 50, 1975–1985. [Google Scholar] [CrossRef]
- Graham, R.S. Understanding flow-induced crystallization in polymers: A perspective on the role of molecular simulations. J. Rheol. 2019, 63, 203–214. [Google Scholar] [CrossRef]
- Jabbarzadeh, A.; Tanner, R. Crystallization of alkanes under quiescent and shearing conditions. J. Non-Newton. Fluid Mech. 2009, 160, 11–21. [Google Scholar] [CrossRef]
- Jabbarzadeh, A.; Tanner, R.I. Flow-induced crystallization: Unravelling the effects of shear rate and strain. Macromolecules 2010, 43, 8136–8142. [Google Scholar] [CrossRef]
- Jabbarzadeh, A.; Chen, X. Surface induced crystallization of polymeric nano-particles: Effect of surface roughness. Faraday Discuss. 2017, 204, 307–330. [Google Scholar] [CrossRef]
- Cacciuto, A.; Auer, S.; Frenkel, D. Onset of heterogeneous crystal nucleation in colloidal suspensions. Nature 2004, 428, 404. [Google Scholar] [CrossRef]
- Ilja Siepmann, B.J.; Martin, M.G. Intermolecular potentials for branched alkanes and the vapour-liquid phase equilibria of n-heptane, 2-methylhexane, and 3-ethylpentane. Mol. Phys. 1997, 90, 687–694. [Google Scholar] [CrossRef]
- Smit, B.; Karaborni, S.; Siepmann, J.I. Computer simulations of vapor–liquid phase equilibria of n-alkanes. J. Chem. Phys. 1995, 102, 2126–2140. [Google Scholar] [CrossRef]
- Ramin, L.; Jabbarzadeh, A. Frictional properties of two alkanethiol self assembled monolayers in sliding contact: Odd-even effects. J. Chem. Phys. 2012, 137, 174706. [Google Scholar] [CrossRef]
- Ramin, L.; Jabbarzadeh, A. Effect of water on structural and frictional properties of self assembled monolayers. Langmuir 2013, 29, 13367–13378. [Google Scholar] [CrossRef]
- Evans, D.; Morriss, G. Statistical Mechanics of Nonequilibrium Liquids; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Daivis, P.J.; Evans, D.J. Comparison of constant pressure and constant volume nonequilibrium simulations of sheared model decane. J. Chem. Phys. 1994, 100, 541–547. [Google Scholar] [CrossRef]
- Yaws, C.L. Thermophysical Properties of Chemicals and Hydrocarbons; William Andrew Publishing: Norwich, NY, USA, 2009. [Google Scholar]
- Shashikanth, P.; Prasad, P. Intrachain defects in n-C60H122 hydrocarbon: A low angle powder XRD study. Curr. Sci. 2001, 80, 1578–1581. [Google Scholar]
- Jabbarzadeh, A. Molecular dynamics simulation of thin liquid films. Ph.D. Thesis, University of Sydney, 1997. [Google Scholar]
- Jabbarzadeh, A.; Harrowell, P.; Tanner, R.I. Crystal bridge formation marks the transition to rigidity in a thin lubrication film. Phys. Rev. Lett. 2006, 96, 206102. [Google Scholar] [CrossRef] [PubMed]
- Avrami, M. Kinetics of phase change. I General theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Wang, Z.; Ju, J.; Yang, J.; Ma, Z.; Liu, D.; Cui, K.; Li, L. The non-equilibrium phase diagrams of flow-induced crystallization and melting of polyethylene. Sci. Rep. 2016, 6, 32968. [Google Scholar] [CrossRef]
- Wang, H.; Keum, J.K.; Hiltner, A.; Baer, E. Crystallization kinetics of poly (ethylene oxide) in confined nanolayers. Macromolecules 2010, 43, 3359–3364. [Google Scholar] [CrossRef]







| Cubic Nanoparticles (D = 4.5 nm) | ||||||
|---|---|---|---|---|---|---|
| Volume Fraction (%) | Cooling Stage | Enhanced Crystallization | Retarded Crystallization | |||
| lnK(T)(s−n) | n | lnK(T)(s−n) | n | lnK(T)(s−n) | n | |
| 2.33 | 33.34 | 1.72 | 4.56 | 0.35 | 12.90 | 0.81 |
| 3.21 | 24.90 | 1.35 | 4.70 | 0.35 | 12.29 | 0.77 |
| 4.56 | 25.68 | 1.39 | 3.71 | 0.30 | 11.35 | 0.72 |
| 6.81 | 33.72 | 1.77 | 3.34 | 0.31 | 10.17 | 0.65 |
| 10.83 | 28.29 | 1.49 | 3.45 | 0.27 | 7.98 | 0.52 |
| 19.27 | 11.52 | 0.68 | 3.27 | 0.26 | 7.36 | 0.50 |
| 0 (Pure Polymer) | 41.4 | 2.17 | 4.49 | 0.344 | 15.25 | 0.94 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabbarzadeh, A. The Origins of Enhanced and Retarded Crystallization in Nanocomposite Polymers. Nanomaterials 2019, 9, 1472. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9101472
Jabbarzadeh A. The Origins of Enhanced and Retarded Crystallization in Nanocomposite Polymers. Nanomaterials. 2019; 9(10):1472. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9101472
Chicago/Turabian StyleJabbarzadeh, Ahmad. 2019. "The Origins of Enhanced and Retarded Crystallization in Nanocomposite Polymers" Nanomaterials 9, no. 10: 1472. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9101472