Dose-Related Antihypertensive Properties and the Corresponding Mechanisms of a Chicken Foot Hydrolysate in Hypertensive Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Chicken Foot Hydrolysate Hpp11: Obtainment and Characterisation
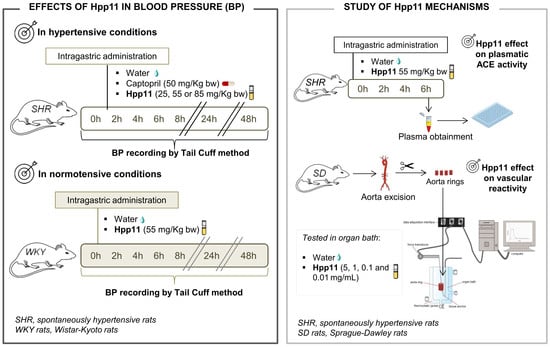
2.3. Experimental Procedure in the SHR and WKY Rats
2.4. Determination of the Plasmatic ACE Activity
2.5. Experiments in Aorta Rings
2.6. Statistical Analysis
3. Results
3.1. Chicken Foot Hydrolysate Hpp11
3.2. Hpp11 In Vitro Inhibition Pattern on ACE
3.3. Effect of Different Doses of Hpp11 on Blood Pressure in Hypertensive and Normotensive Rats
3.4. Mechanisms Involved in the Antihypertensive Effect of Hpp11
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Messerli, F.H.; Williams, B.; Ritz, E. Essential hypertension. Lancet 2007, 370, 591–603. [Google Scholar] [CrossRef]
- Hedayati, S.; Elsayed, E.; Reilly, R. Non-pharmacological aspects of blood pressure management: What are the data? Kidney Int. 2011, 79, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Sun, L.; Zhang, Y.; Liu, G. Antihypertensive effect of long-term oral administration of jellyfish (Rhopilema esculentum) collagen peptides on renovascular hypertension. Mar. Drugs 2012, 10, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.Y.; Wan, T.C.; Liu, Y.T.; Chen, C.M.; Lin, L.C.; Sakata, R. Determination of angiotensin-I converting enzyme inhibitory peptides in chicken leg bone protein hydrolysate with alcalase. Anim. Sci. J. 2009, 80, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Sica, D.A. Angiotensin-converting enzyme inhibitors side effects—Physiologic and non-physiologic considerations. J. Clin. Hypertens. 2004, 6, 410–416. [Google Scholar] [CrossRef]
- Margalef, M.; Bravo, F.I.; Arola-Arnal, A.; Muguerza, B. Natural angiotensin converting enzyme (ACE) inhibitors with antihypetensive properties. In Natural Products Targeting Clinically Relevant Enzymes; Andrade, P., Valentao, P., Pereira, D.M., Eds.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2017; pp. 45–67. [Google Scholar]
- Iwaniak, A.; Minkiewicz, P.; Darewicz, M. Food-originating ACE inhibitors, including antihypertensive peptides, as preventive food components in blood pressure reduction. Compr. Rev. Food Sci. Food Saf. 2014, 13, 114–134. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Wu, J. Bioactive peptides on endothelial function. Food Sci. Hum. Wellness 2016, 5, 1–7. [Google Scholar] [CrossRef]
- Fang, H.; Luo, M.; Sheng, Y.; Li, Z.; Wu, Y.; Liu, C. The antihypertensive effect of peptides: A novel alternative to drugs? Peptides 2008, 29, 1062–1071. [Google Scholar]
- Martínez-Maqueda, D.; Miralles, B.; Recio, I.; Hernández-Ledesma, B. Antihypertensive peptides from food proteins: A review. Food Funct. 2012, 3, 350. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre, A.; Miguel, M.; Muguerza, B. Péptidos antihipertensivos derivados de proteínas de leche y huevo. Nutr. Hosp. 2008, 23, 313–318. [Google Scholar] [PubMed]
- Huang, J.; Liu, Q.; Xue, B.; Chen, L.; Wang, Y.; Ou, S.; Peng, X. Angiotensin-I-converting enzyme inhibitory activities and in vivo Antihypertensive Effects of Sardine Protein Hydrolysate. J. Food Sci. 2016, 81, H2831–H2840. [Google Scholar] [CrossRef] [PubMed]
- Slizyte, R.; Rommi, K.; Mozuraityte, R.; Eck, P.; Five, K.; Rustad, T. Bioactivities of fish protein hydrolysates from defatted salmon backbones. Biotechnol. Rep. 2016, 11, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Ahhmed, A.M.; Muguruma, M. A review of meat protein hydrolysates and hypertension. Meat Sci. 2010, 86, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Ketnawa, S.; Rawdkuen, S. Angiotensin converting enzyme inhibitory peptides from aquatic and their processing by-Products: A review. Int. J. Sci. Innov. Discov. 2013, 2, 185–199. [Google Scholar]
- Karamaæ, M.; Flaczyk, E.; Wanasundara, P.K.J.P.D.; Amarowicz, R. Angiotensin I-converting enzyme (ACE) inhibitory activity of hydrolysates obtained from muscle food industry by-products—A short report. Pol. J. Food Nutr. Sci. 2005, 14, 133–137. [Google Scholar]
- Mora, L.; Reig, M.; Toldrá, F. Bioactive peptides generated from meat industry by-products. Food Res. Int. 2014, 65, 344–349. [Google Scholar] [CrossRef] [Green Version]
- Bravo, F.I.; Arola, L.; Muguerza, B. Procedure for obtaining a hydrolysate claw chicken leg with antihypertensive activity, and peptides obtained hydrolysate containing. Patent No. ES2606954B1, 11 December 2017. [Google Scholar]
- Lasekan, A.; Abu Bakar, F.; Hashim, D. Potential of chicken by-products as sources of useful biological resources. Waste Manag. 2013, 33, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Toldrá, F.; Aristoy, M.C.; Mora, L.; Reig, M. Innovations in value-addition of edible meat by-products. Meat Sci. 2012, 92, 290–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onuh, J.O.; Girgih, A.T.; Aluko, R.E.; Aliani, M. Inhibitions of renin and angiotensin converting enzyme activities by enzymatic chicken skin protein hydrolysates. Food Res. Int. 2013, 53, 260–267. [Google Scholar] [CrossRef]
- Onuh, J.O.; Girgih, A.T.; Malomo, S.A.; Aluko, R.E.; Aliani, M. Kinetics of in vitro renin and angiotensin converting enzyme inhibition by chicken skin protein hydrolysates and their blood pressure lowering effects in spontaneously hypertensive rats. J. Funct. Foods 2015, 14, 133–143. [Google Scholar] [CrossRef]
- Onuh, J.O.; Girgih, A.T.; Nwachukwu, I.; Ievari-Shariati, S.; Raj, P.; Netticadan, T.; Aluko, R.E.; Aliani, M. A metabolomics approach for investigating urinary and plasma changes in spontaneously hypertensive rats (SHR) fed with chicken skin protein hydrolysates diets. J. Funct. Foods 2016, 22, 20–33. [Google Scholar] [CrossRef]
- Majumder, K.; Wu, J. Molecular Targets of Antihypertensive Peptides: Understanding the mechanisms of action based on the pathophysiology of hypertension. Int. J. Mol. Sci. 2015, 16, 256–283. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.; Manso, M.; Aleixandre, A.; Alonso, M.J.; Salaices, M.; Lopez-Fandino, R. Vascular effects, angiotensin I-converting enzyme (ACE)-inhibitory activity, and antihypertensive properties of peptides derived from egg white. J. Agric. Food Chem. 2007, 55, 10615–10621. [Google Scholar] [CrossRef] [PubMed]
- Girgih, A.T.; Nwachukwu, I.D.; Onuh, J.O.; Malomo, S.A.; Aluko, R.E. Antihypertensive properties of a pea protein hydrolysate during short- and long-term oral administration to spontaneously hypertensive rats. J. Food Sci. 2016, 81, H1281–H1287. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-C.; Alashi, A.M.; Aluko, R.E.; Sun Pan, B.; Chang, Y.-W. Antihypertensive properties of tilapia (Oreochromis spp.) frame and skin enzymatic protein hydrolysates. Food Nutr. Res. 2017, 61, 1391666. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.; Alonso, M.J.; Salaices, M.; Aleixandre, A.; López-Fandiño, R. Antihypertensive, ACE-inhibitory and vasodilator properties of an egg white hydrolysate: Effect of a simulated intestinal digestion. Food Chem. 2007, 104, 163–168. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC) (Ed.) Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Quirós, A.; del Contreras, M.M.; Ramos, M.; Amigo, L.; Recio, I. Stability to gastrointestinal enzymes and structure–activity relationship of β-casein-peptides with antihypertensive properties. Peptides 2009, 30, 1848–1853. [Google Scholar] [CrossRef] [PubMed]
- Buñag, R.D. Validation in awake rats of a tail-cuff method for measuring systolic pressure. J. Appl. Physiol. 1973, 34, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N. Antihypertensive peptides derived from food proteins. Biopolym. Pept. Sci. Sect. 1997, 43, 129–134. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Mohan, A. Mechanisms of food protein-derived antihypertensive peptides other than ACE inhibition. J. Funct. Foods 2014, 8, 45–52. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, Z.; Dai, Z.; Wang, G.; Wu, G. Protein hydrolysates in animal nutrition: Industrial production, bioactive peptides, and functional significance. J. Anim. Sci. Biotechnol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Saiga, A.; Iwai, K.; Hayakawa, T.; Takahata, Y.; Kitamura, S.; Nishimura, T.; Morimatsu, F. Angiotensin I-converting enzyme-inhibitory peptides obtained from chicken collagen hydrolysate. J. Agric. Food Chem. 2008, 56, 9586–9591. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Therkildsen, M.; Aluko, R.E.; Lametsch, R. Exploration of collagen recovered from animal by-products as a precursor of bioactive peptides: Successes and challenges. Crit. Rev. Food Sci. Nutr. 2018, 8398, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Balti, R.; Bougatef, A.; Ali, N.E.H.; Zekri, D.; Barkia, A.; Nasri, M. Influence of degree of hydrolysis on functional properties and angiotensin I-converting enzyme-inhibitory activity of protein hydrolysates from cuttlefish (Sepia officinalis) by-products. J. Sci. Food Agric. 2010, 90, 2006–2014. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.Y.; Wan, T.C.; Liu, Y.T.; Lai, K.M.; Lin, L.C.; Sakata, R. A study of in vivo antihypertensive properties of enzymatic hydrolysate from chicken leg bone protein. Anim. Sci. J. 2008, 79, 614–619. [Google Scholar] [CrossRef]
- Li, G.-H.; Qu, M.-R.; Wan, J.-Z.; You, J.-M. Antihypertensive effect of rice protein hydrolysate with in vitro angiotensin I-converting enzyme inhibitory activity in spontaneously hypertensive rats. Asia Pac. J. Clin. Nutr. 2007, 16 (Suppl. 1), 275–280. [Google Scholar]
- Vermeirssen, V.; Van Camp, J.; Verstraete, W. Bioavailability of angiotensin I converting enzyme inhibitory peptides. Br. J. Nutr. 2004, 92, 357. [Google Scholar] [CrossRef] [PubMed]
- Jao, C.-L.; Huang, S.-L.; Hsu, K.-C. Angiotensin I-converting enzyme inhibitory peptides: Inhibition mode, bioavailability, and antihypertensive effects. BioMedicine 2012, 2, 130–136. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Girgih, A.T.; Mohan, A.; Gong, M.; Malomo, S.A.; Aluko, R.E. Antihypertensive and bovine plasma oxidation-inhibitory activities of spent hen meat protein hydrolysates. J. Food Biochem. 2017, 41, 1–8. [Google Scholar] [CrossRef]
- Yoshii, H.; Tachi, N.; Ohba, R.; Sakamura, O.; Takeyama, H.; Itani, T. Antihypertensive effect of ACE inhibitory oligopeptides from chicken egg yolks. Comp. Biochem. Physiol. C 2001, 128, 27–33. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Yang, S.-C.; Chen, J.-R.; Tzeng, Y.-H.; Han, B.-C. Soyabean protein hydrolysate prevents the development of hypertension in spontaneously hypertensive rats. Br. J. Nutr. 2004, 92, 507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quirós, A.; Ramos, M.; Muguerza, B.; Delgado, M.A.; Miguel, M.; Aleixandre, A.; Recio, I. Identification of novel antihypertensive peptides in milk fermented with Enterococcus faecalis. Int. Dairy J. 2007, 17, 33–41. [Google Scholar] [CrossRef]
- Alderman, C.P. Adverse effects of the angiotensin-converting enzyme inhibitors. Ann Pharmacoter. 1996, 30, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Sipola, M.; Finckenberg, P.; Vapaatalo, H.; Pihlanto-Leppälä, A.; Korhonen, H.; Korpela, R.; Nurminen, M.-L. Alpha-lactorphin and beta-lactorphin improve arterial function in spontaneously hypertensive rats. Life Sci. 2002, 71, 1245–1253. [Google Scholar] [CrossRef]
- Fujita, H.; Suganuma, H.; Usui, H.; Kurahashi, K.; Nakagiri, R.; Sasaki, R.; Yoshikawa, M. Vasorelaxation by casomokinin L, a derivative of β-casomorphin and casoxin D, is mediated by NK1receptor. Peptides 1996, 17, 635–639. [Google Scholar] [CrossRef]
- Rahmani, M.A.; DeGray, G.; David, V.; Ampy, F.R.; Jones, L. Comparison of calcium import as a function of contraction in the aortic smooth muscle of Sprague-Dawley, Wistar Kyoto and spontaneously hypertensive rats. Front. Biosci. 1999, 4, D408–D415. [Google Scholar] [PubMed]
- Pons, Z.; Arola, L. Involvement of nitric oxide and prostacyclin in the antihypertensive effect of low-molecular-weight procyanidin rich grape seed extract in male spontaneously hypertensive rats. J. Funct. Foods 2014, 6, 419–427. [Google Scholar]





| Determinations | (%) |
|---|---|
| Total protein content a | 0.67 |
| Humidity | 98.93 |
| Ash content b | 0.17 |
| Degree of hydrolysis c | 18.85 |
| ACE inhibition d | 95.11 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mas-Capdevila, A.; Pons, Z.; Aleixandre, A.; Bravo, F.I.; Muguerza, B. Dose-Related Antihypertensive Properties and the Corresponding Mechanisms of a Chicken Foot Hydrolysate in Hypertensive Rats. Nutrients 2018, 10, 1295. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10091295
Mas-Capdevila A, Pons Z, Aleixandre A, Bravo FI, Muguerza B. Dose-Related Antihypertensive Properties and the Corresponding Mechanisms of a Chicken Foot Hydrolysate in Hypertensive Rats. Nutrients. 2018; 10(9):1295. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10091295
Chicago/Turabian StyleMas-Capdevila, Anna, Zara Pons, Amaya Aleixandre, Francisca I. Bravo, and Begoña Muguerza. 2018. "Dose-Related Antihypertensive Properties and the Corresponding Mechanisms of a Chicken Foot Hydrolysate in Hypertensive Rats" Nutrients 10, no. 9: 1295. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10091295