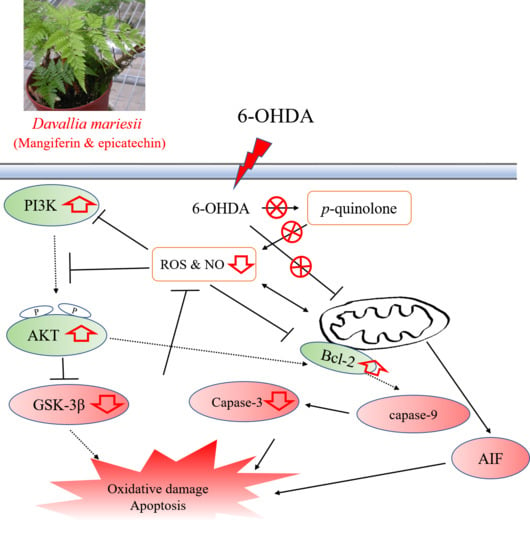
Aqueous Extract of Davallia mariesii Attenuates 6-Hydroxydopamine-Induced Oxidative Damage and Apoptosis in B35 Cells Through Inhibition of Caspase Cascade and Activation of PI3K/AKT/GSK-3β Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Collection and Preparation
2.2. Chemicals
2.3. Determination of Antioxidant Phytoconstituents by a Spectrophotometric Reader
2.4. Determination of Phenolic Compounds by HPLC-DAD
2.5. Determination of Radical Scavenging Activity in Vitro
2.6. Inhibition of Lipid Peroxidation In Vitro
2.7. Inhibition of 6-OHDA Autoxidation In Vitro
2.8. Cell Culture and Treatment
2.9. Cell Viability
2.10. Detection of Morphological Changes and Cell Death
2.11. Measurement of Intracellular ROS and NO levels
2.12. Measurement of Caspase-3 Activity
2.13. Biochemical Assays
2.14. Western Blotting
2.15. Statistical Analysis
3. Results
3.1. Antioxidant Phytoconstituents Contents
3.2. Radical Scavenging Capacities
3.3. Phytoconstituent Profiles of DM by HPLC-DAD
3.4. Inhibition of 6-OHDA Autoxidation
3.5. Protection Against 6-OHDA Toxicology in B35 Cells
3.6. Cell Morphology and Apoptosis
3.7. Intracellular ROS and NO Production
3.8. Intracellular Aantioxidant Activities and Oxidative Damage
3.9. Caspase Cascade Pathway
3.10. PI3K/AKT/GSK-3β Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Dexter, D.T.; Jenner, P. Parkinson disease: from pathology to molecular disease mechanisms. Free Radic. Biol. Med. 2013, 62, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s disease: mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Biosa, A.; Arduini, I.; Soriano, M.E.; Giorgio, V.; Bernardi, P.; Bisaglia, M.; Bubacco, L. Dopamine oxidation products as mitochondrial endotoxins, a potential molecular mechanism for preferential neurodegeneration in Parkinson’s disease. ACS Chem. Neurosci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.D.; Zhao, X.; Li, Y.; Li, G.R.; Liu, X.L. Damage to dopaminergic neurons by oxidative stress in Parkinson’s disease. Int. J. Mol. Med. 2018, 41, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Perier, C.; Bove, J.; Vila, M. Mitochondria and programmed cell death in Parkinson’s disease: apoptosis and beyond. Antioxid. Redox Signal. 2012, 16, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Bastias-Candia, S.; Zolezzi, J.M.; Inestrosa, N.C. Revisiting the paraquat-induced sporadic Parkinson’s disease-like model. Mol. Neurobiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Baltazar, D.; Mendoza-Garrido, M.E.; Martinez-Fong, D. Activation of GSK-3β and caspase-3 occurs in Nigral dopamine neurons during the development of apoptosis activated by a striatal injection of 6-hydroxydopamine. PLoS One 2013, 8, e70951. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.K.; Qiao, L.Y.; Wen, X.N. Safranal prevents rotenone-induced oxidative stress and apoptosis in an in vitro model of Parkinson’s disease through regulating Keap1/Nrf2 signaling pathway. Cell. Mol. Biol. 2016, 62, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; Gao, L.; Wang, X.; Ding, X.; Teng, J. Intranasal administration of GDNF protects against neural apoptosis in a rat model of Parkinson’s disease through PI3K/Akt/GSK3β pathway. Neurochem. Res. 2017, 42, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Li, D.W.; Liu, Z.Q.; Chen, W.; Yao, M.; Li, G.R. Association of glycogen synthase kinase-3β with Parkinson’s disease (review). Mol. Med. Rep. 2014, 9, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Golpich, M.; Amini, E.; Hemmati, F.; Ibrahim, N.M.; Rahmani, B.; Mohamed, Z.; Raymond, A.A.; Dargahi, L.; Ghasemi, R.; Ahmadiani, A. Glycogen synthase kinase-3 beta (GSK-3β) signaling: Implications for Parkinson’s disease. Pharmacol. Res. 2015, 97, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cheng, L.; Wei, X.; Yuan, Z.; Wu, Y.; Wang, S.; Ren, Z.; Liu, X.; Liu, H. Tetramethylpyrazine analogue CXC195 protects against dopaminergic neuronal apoptosis via activation of PI3K/Akt/GSK3β signaling pathway in 6-OHDA-induced Parkinson’s disease mice. Neurochem. Res. 2017, 42, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Qan, Q. Research advances in chemical constituents and pharmacological activities of Rhizoma Drynariaed. Chin. J. Biochem. Pharma. 2015, 35, 186–188. [Google Scholar]
- Kuo, H.C.; Chang, H.C.; Lan, W.C.; Tsai, F.H.; Liao, J.C.; Wu, C.R. Protective effects of Drynaria fortunei against 6-hydroxydopamine-induced oxidative damage in B35 cells via the PI3K/AKT pathway. Food Funct. 2014, 5, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Huang, G.J.; Agrawal, D.C.; Kuo, C.L.; Wu, C.R.; Tsay, H.S. Antioxidant activities and polyphenol contents of six folk medicinal ferns used as "Gusuibu". Bot. Stud. 2007, 48, 397–406. [Google Scholar]
- Lin, C.H.; Wu, J.B.; Jian, J.Y.; Shih, C.C. (-)-Epicatechin-3-O-β-D-allopyranoside from Davallia formosana prevents diabetes and dyslipidemia in streptozotocin-induced diabetic mice. PLoS One 2017, 12, e0173984. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.F.; Lin, Y.S.; Lee, S.C.; Chen, C.Y.; Wu, M.C.; Lin, J.S. Effects of Davallia formosana Hayata water and alcohol extracts on osteoblastic MC3T3-E1 cells. Phytother. Res. 2017, 31, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.R.; Lin, W.H.; Hseu, Y.C.; Lien, J.C.; Lin, Y.T.; Kuo, T.P.; Ching, H. Evaluation of the antioxidant activity of five endemic Ligustrum species leaves from Taiwan flora in vitro. Food Chem. 2011, 127, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.Y.; Chen, S.C.; Wu, K.J.; Kuo, H.C.; Hseu, Y.C.; Ching, H.; Wu, C.R. Antioxidant phenolic profile from ethyl acetate fraction of Fructus Ligustri Lucidi with protection against hydrogen peroxide-induced oxidative damage in SH-SY5Y cells. Food Chem. Toxicol. 2012, 50, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Peng, Y.; Huang, K.; Lei, Y.; Liu, H.L.; Tao, Y.Y.; Liu, C.H. Salvianolate protects hepatocytes from oxidative stress by attenuating mitochondrial injury. Evid. Based Complement. Alternat. Med. 2016, 2016, 5408705. [Google Scholar] [CrossRef] [PubMed]
- Tjalkens, R.B.; Carbone, D.L.; Wu, G. Detection of nitric oxide formation in primary neural cells and tissues. Methods Mol. Biol. 2011, 758, 267–277. [Google Scholar] [PubMed]
- Sang, S.; Cheng, X.; Stark, R.E.; Rosen, R.T.; Yang, C.S.; Ho, C.T. Chemical studies on antioxidant mechanism of tea catechins: analysis of radical reaction products of catechin and epicatechin with 2,2-diphenyl-1-picrylhydrazyl. Bioorg. Med. Chem. 2002, 10, 2233–2237. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.; Sawano, T.; Ito, H. Antioxidative properties of vanillic acid esters in multiple antioxidant assays. Biosci. Biotechnol. Biochem. 2012, 76, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Matkowski, A.; Kus, P.; Goralska, E.; Wozniak, D. Mangiferin - a bioactive xanthonoid, not only from mango and not just antioxidant. Mini Rev. Med. Chem. 2013, 13, 439–455. [Google Scholar] [PubMed]
- Kalia, L.V.; Kalia, S.K.; Lang, A.E. Disease-modifying strategies for Parkinson’s disease. Mov. Disord. 2015, 30, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; Carvalho, A.C.; Trevisan, M.T.; Andrade, G.M.; Nobre-Junior, H.V.; Moraes, M.O.; Magalhaes, H.I.; Morais, T.C.; Santos, F.A. Mangiferin ameliorates 6-hydroxydopamine-induced cytotoxicity and oxidative stress in ketamine model of schizophrenia. Pharmacol. Rep. 2012, 64, 848–856. [Google Scholar] [CrossRef]
- Alberdi, E.; Sanchez-Gomez, M.V.; Ruiz, A.; Cavaliere, F.; Ortiz-Sanz, C.; Quintela-Lopez, T.; Capetillo-Zarate, E.; Sole-Domenech, S.; Matute, C. Mangiferin and morin attenuate oxidative stress, mitochondrial dysfunction, and neurocytotoxicity, induced by amyloid beta oligomers. Oxid. Med. Cell. Longev. 2018, 2018, 2856063. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, S.; Wang, J.; Shen, Y.; Zhang, J.; Wu, Q. Nrf2/HO-1 mediates the neuroprotective effect of mangiferin on early brain injury after subarachnoid hemorrhage by attenuating mitochondria-related apoptosis and neuroinflammation. Sci. Rep. 2017, 7, 11883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavitha, M.; Manivasagam, T.; Essa, M.M.; Tamilselvam, K.; Selvakumar, G.P.; Karthikeyan, S.; Thenmozhi, J.A.; Subash, S. Mangiferin antagonizes rotenone: induced apoptosis through attenuating mitochondrial dysfunction and oxidative stress in SK-N-SH neuroblastoma cells. Neurochem. Res. 2014, 39, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, M.; Nataraj, J.; Essa, M.M.; Memon, M.A.; Manivasagam, T. Mangiferin attenuates MPTP induced dopaminergic neurodegeneration and improves motor impairment, redox balance and Bcl-2/Bax expression in experimental Parkinson’s disease mice. Chem. Biol. Interact. 2013, 206, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Bitu Pinto, N.; da Silva Alexandre, B.; Neves, K.R.; Silva, A.H.; Leal, L.K.; Viana, G.S. Neuroprotective properties of the standardized extract from Camellia sinensis (green Tea) and its main bioactive components, epicatechin and epigallocatechin gallate, in the 6-OHDA model of Parkinson’s disease. Evid. Based Complement. Alternat. Med. 2015, 2015, 161092. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, E.; Limon, D.; Perez-Severiano, F.; Diaz, A.; Ortega, L.; Zenteno, E.; Guevara, J. Antioxidant effects of epicatechin on the hippocampal toxicity caused by amyloid-beta 25-35 in rats. Eur. J. Pharmacol. 2009, 616, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Porras, A.R.; Arevalo-Arbelaez, A.; Alzate-Restrepo, J.F.; Velez-Pardo, C.; Jimenez-Del-Rio, M. PARKIN overexpression in human mesenchymal stromal cells from Wharton’s jelly suppresses 6-hydroxydopamine-induced apoptosis: Potential therapeutic strategy in Parkinson’s disease. Cytotherapy 2018, 20, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Nakano, N.; Matsuda, S.; Ichimura, M.; Minami, A.; Ogino, M.; Murai, T.; Kitagishi, Y. PI3K/AKT signaling mediated by G protein-coupled receptors is involved in neurodegenerative Parkinson’s disease (Review). Int. J. Mol. Med. 2017, 39, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H. Targeting the NF-E2-related factor 2 pathway: a novel strategy for traumatic brain injury. Mol. Neurobiol. 2018, 55, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, S.; Sadhukhan, P.; Sinha, K.; Agarwal, N.; Sil, P.C. Mangiferin attenuates oxidative stress induced renal cell damage through activation of PI3K induced Akt and Nrf-2 mediated signaling pathways. Biochem. Biophys. Rep. 2016, 5, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Weian, C.; Susu, H.; Hanmin, W. Protective effects of mangiferin on cerebral ischemia-reperfusion injury and its mechanisms. Eur. J. Pharmacol. 2016, 771, 145–151. [Google Scholar] [CrossRef] [PubMed]
- De Los Santos, S.; Garcia-Perez, V.; Hernandez-Resendiz, S.; Palma-Flores, C.; Gonzalez-Gutierrez, C.J.; Zazueta, C.; Canto, P.; Coral-Vazquez, R.M. (-)-Epicatechin induces physiological cardiac growth by activation of the PI3K/Akt pathway in mice. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Wang, X.Y.; Zhang, X.; Gao, L.; Wang, L.F.; Yin, X.H. (-)-Epicatechin protects against myocardial ischemia induced cardiac injury via activation of the PTEN/PI3K/AKT pathway. Mol. Med. Rep. 2018, 17, 8300–8308. [Google Scholar] [CrossRef] [PubMed]
- Sadhukhan, P.; Saha, S.; Dutta, S.; Sil, P.C. Mangiferin ameliorates cisplatin induced acute kidney injury by upregulating Nrf-2 via the activation of PI3K and exhibits synergistic anticancer activity with cisplatin. Front. Pharmacol. 2018, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Huttemann, M. Molecular mechanisms and therapeutic effects of (-)-epicatechin and other polyphenols in cancer, inflammation, diabetes, and neurodegeneration. Oxid. Med. Cell. Longev. 2015, 2015, 181260. [Google Scholar] [CrossRef] [PubMed]
- Bernatova, I. Biological activities of (-)-epicatechin and (-)-epicatechin-containing foods: Focus on cardiovascular and neuropsychological health. Biotechnol. Adv. 2018, 36, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Ban, J.Y.; Jeon, S.Y.; Bae, K.; Song, K.S.; Seong, Y.H. Catechin and epicatechin from Smilacis chinae rhizome protect cultured rat cortical neurons against amyloid beta protein (25-35)-induced neurotoxicity through inhibition of cytosolic calcium elevation. Life Sci. 2006, 79, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Nichols, M.; Zhang, J.; Polster, B.M.; Elustondo, P.A.; Thirumaran, A.; Pavlov, E.V.; Robertson, G.S. Synergistic neuroprotection by epicatechin and quercetin: Activation of convergent mitochondrial signaling pathways. Neuroscience 2015, 308, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Pedrielli, P.; Skibsted, L.H. Antioxidant synergy and regeneration effect of quercetin, (-)-epicatechin, and (+)-catechin on alpha-tocopherol in homogeneous solutions of peroxidating methyl linoleate. J. Agric. Food Chem. 2002, 50, 7138–7144. [Google Scholar] [CrossRef] [PubMed]








| Samples | Total Phenolics (mg of catechin/g) | Flavonols (mg of quercetin/g) | Phenylpropanoids (mg of verbascoside/g) | Anthocyanidin (mg of cyanidin/g) |
|---|---|---|---|---|
| AP | 231.26 ± 5.74 | 13.70 ± 0.20 | 152.87 ± 3.95 | 2.06 ± 0.08 |
| DF | 141.25 ± 8.65 | 6.26 ± 0.18 | 124.37 ± 6.23 | 1.28 ± 0.10 |
| DG | 18.28 ± 1.58 | 12.24 ± 0.18 | 14.82 ± 1.56 | 1.55 ± 0.03 |
| DM | 267.02 ± 4.99 | 11.81 ± 0.26 | 170.06 ± 9.73 | 2.67 ± 0.26 |
| DS | 172.95 ± 3.17 | 13.88 ± 0.21 | 87.41 ± 6.27 | 0.68 ± 0.03 |
| Samples | DPPH Scavenging (mg of catechin/g) | O2˙ Scavenging (U of SOD/mg) | H2O2 Scavenging (μmol of trolox/g) | OH˙ Scavenging (mg of quercetin/g) | IC50 of Lipid Peroxidation Inhibition (mg/mL) | FRAP (mmol of trolox/g) |
|---|---|---|---|---|---|---|
| AP | 391.32 ± 43.40 | 23.11 ± 2.97 | 1607.27 ± 19.82 | 19.66 ± 3.25 | 21.53 ± 0.95 | 935.76 ± 17.73 |
| DF | 166.80 ± 1.76 | 11.58 ± 0.70 | 831.12 ± 50.71 | 23.47 ± 2.04 | 126.44 ± 15.30 | 695.34 ± 22.63 |
| DG | 11.84 ± 0.49 | 1.25 ± 0.07 | 8.43 ± 0.30 | 3.74 ± 0.28 | 156.40 ± 12.88 | 710.29 ± 15.53 |
| DM | 264.38 ± 5.95 | 18.57 ± 1.79 | 1730.29 ± 70.28 | 23.79 ± 4.88 | 31.90 ± 3.76 | 1066.46 ± 41.43 |
| DS | 211.31 ± 1.29 | 19.93 ± 0.81 | 1025.77 ± 17.59 | 13.30 ± 2.52 | 38.02 ± 3.01 | 674.78 ± 25.88 |
| DPPH | O2˙ | H2O2 | OH˙ | LPO | FRAP | TP | TF | TPP | |
|---|---|---|---|---|---|---|---|---|---|
| O2˙ | 0.94 * | ||||||||
| H2O2 | 0.92 * | 0.91 * | |||||||
| OH˙ | 0.68 | 0.64 | 0.80 | ||||||
| LPO | 0.93 * | 0.88 * | 0.85 | 0.41 | |||||
| FRAP | 0.63 | 0.50 | 0.78 | 0.54 | 0.68 | ||||
| TP | 0.89 * | 0.91 * | 0.996 ** | 0.81 | 0.81 | 0.75 | |||
| TF | 0.29 | 0.36 | 0.21 | −0.42 | 0.62 | 0.25 | 0.18 | ||
| TPP | 0.86 | 0.80 | 0.95 * | 0.94 * | 0.67 | 0.73 | 0.95* | −0.11 | |
| TA | 0.39 | 0.19 | 0.54 | 0.41 | 0.44 | 0.94* | 0.51 | 0.07 | 0.56 |
| Samples | GSH (pmol/mg of protein) | GR (mU/mg of protein) | GPx (mU/mg of protein) | MDA (nmol/mg of protein) |
|---|---|---|---|---|
| Control | 6.61 ± 0.16 ** | 14.45 ± 0.76 ** | 157.22 ± 6.19 *** | 8.80 ± 0.21** |
| 6-OHDA | 2.84 ± 0.12 | 9.78 ± 0.46 | 71.79 ± 6.82 | 15.47 ± 1.70 |
| DM 5 μg/mL + 6-OHDA | 2.77 ± 0.08 | 9.74 ± 0.45 | 73.89 ± 5.77 | 12.18 ± 0.14 |
| DM 10 μg/mL + 6-OHDA | 3.68 ± 0.12 | 12.00 ± 0.56 | 106.95 ± 7.39 ** | 10.92 ± 1.34 |
| DM 50 μg/mL + 6-OHDA | 4.50 ± 0.16 * | 12.87 ± 0.50 * | 119.57 ± 5.37 ** | 10.74 ± 0.21 * |
| DM 100 μg/mL + 6-OHDA | 4.68 ± 0.13 * | 14.05 ± 0.04 ** | 149.64 ± 9.54 *** | 9.84 ± 0.50 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-R.; Chang, H.-C.; Cheng, Y.-D.; Lan, W.-C.; Yang, S.-E.; Ching, H. Aqueous Extract of Davallia mariesii Attenuates 6-Hydroxydopamine-Induced Oxidative Damage and Apoptosis in B35 Cells Through Inhibition of Caspase Cascade and Activation of PI3K/AKT/GSK-3β Pathway. Nutrients 2018, 10, 1449. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10101449
Wu C-R, Chang H-C, Cheng Y-D, Lan W-C, Yang S-E, Ching H. Aqueous Extract of Davallia mariesii Attenuates 6-Hydroxydopamine-Induced Oxidative Damage and Apoptosis in B35 Cells Through Inhibition of Caspase Cascade and Activation of PI3K/AKT/GSK-3β Pathway. Nutrients. 2018; 10(10):1449. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10101449
Chicago/Turabian StyleWu, Chi-Rei, Hung-Chi Chang, Yih-Dih Cheng, Wan-Cheng Lan, Shu-Er Yang, and Hui Ching. 2018. "Aqueous Extract of Davallia mariesii Attenuates 6-Hydroxydopamine-Induced Oxidative Damage and Apoptosis in B35 Cells Through Inhibition of Caspase Cascade and Activation of PI3K/AKT/GSK-3β Pathway" Nutrients 10, no. 10: 1449. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10101449