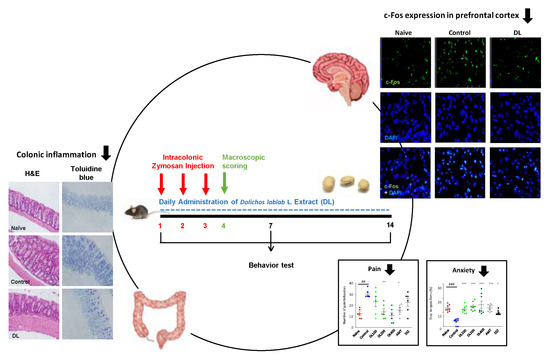
Alleviation of Irritable Bowel Syndrome-Like Symptoms and Control of Gut and Brain Responses with Oral Administration of Dolichos lablab L. in a Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of D. lablab L.
2.2. Standardization of DL
2.3. Animal Experiments
2.4. Macroscopic Scoring of Zymosan-Induced Colon Changes
2.5. Body Weight Changes and Food Intake
2.6. Histological Examination
2.7. Polymerase Chain Reaction (PCR)
2.8. Murine Bone Marrow-Derived Mast Cells Culture
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Pain-Related Behavior Test
2.11. Anxiety-Related Behavior Test
2.12. Immunofluorescence Analysis (IF)
2.13. Western Blotting
2.14. Statistical Analysis
3. Results
3.1. Standardization of DL
3.2. Effects of DL on the Macroscopic Score of Zymosan-Induced Colon Changes
3.3. Effects of DL on Body Weight Changes and Food Intake
3.4. Effects of DL on Zymosan-Induced Colonic Inflammation
3.5. Effects of DL on Visceral Pain-Related Behaviors
3.6. Effects of DL on Anxiety-Like Behaviors
3.7. Effects of DL on c-Fos Protein Expression in the Brain
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chey, W.D.; Kurlander, J.; Eswaran, S. Irritable bowel syndrome: A clinical review. JAMA 2015, 313, 949–958. [Google Scholar] [CrossRef] [PubMed]
- De Ponti, F. Drug development for the irritable bowel syndrome: Current challenges and future perspectives. Front. Pharmacol. 2013, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A. Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; La, J.H.; Schwartz, E.S.; Gebhart, G.F. Irritable bowel syndrome: Methods, mechanisms, and pathophysiology. Neural and neuro-immune mechanisms of visceral hypersensitivity in irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1085–G1098. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Tandon, R.K. Stress and the gastrointestinal tract. J. Gastroenterol. Hepatol. 2005, 20, 332–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbara, G.; De Giorgio, R.; Stanghellini, V.; Cremon, C.; Salvioli, B.; Corinaldesi, R. New pathophysiological mechanisms in irritable bowel syndrome. Aliments Pharmacol. Ther. 2004, 20 (Suppl. 2), 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Sullivan, M.; Clayton, N.; Breslin, N.P.; Harman, I.; Bountra, C.; McLaren, A.; O’Morain, C.A. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol. Motil. 2000, 12, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Piche, T.; Saint-Paul, M.C.; Dainese, R.; Marine-Barjoan, E.; Iannelli, A.; Montoya, M.L.; Peyron, J.F.; Czerucka, D.; Cherikh, F.; Filippi, J.; et al. Mast cells and cellularity of the colonic mucosa correlated with fatigue and depression in irritable bowel syndrome. Gut 2008, 57, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, J.; Hou, X. Mast Cells and Irritable Bowel Syndrome: From the Bench to the Bedside. J. Neurogastroenterol. Motil. 2016, 22, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbara, G.; Stanghellini, V.; De Giorgio, R.; Corinaldesi, R. Functional gastrointestinal disorders and mast cells: Implications for therapy. Neurogastroenterol. Motil. 2006, 18, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Wouters, M.M.; Vicario, M.; Santos, J. The role of mast cells in functional GI disorders. Gut 2016, 65, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, C.S.; Becerra, L.; Heinz, N.; Ludwick, A.; Rasooly, T.; Wu, R.; Johnson, A.; Schechter, N.L.; Borsook, D.; Nurko, S. Abdominal Pain, the Adolescent and Altered Brain Structure and Function. PLoS ONE 2016, 11, e0156545. [Google Scholar] [CrossRef] [PubMed]
- Fukudo, S.; Nomura, T.; Muranaka, M.; Taguchi, F. Brain-gut response to stress and cholinergic stimulation in irritable bowel syndrome. A preliminary study. J. Clin. Gastroenterol. 1993, 17, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Tillisch, K.; Mayer, E.A.; Labus, J.S. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology 2011, 140, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Fond, G.; Loundou, A.; Hamdani, N.; Boukouaci, W.; Dargel, A.; Oliveira, J.; Roger, M.; Tamouza, R.; Leboyer, M.; Boyer, L. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): A systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Al-Snaf, A.E. The pharmacology and medical importance of Dolichos lablab (Lablab purpureus)—A review. Iosrphr 2017, 7, 22–30. [Google Scholar] [CrossRef]
- Habib, H.M.; Theuri, S.W.; Kheadr, E.E.; Mohamed, F.E. Functional, bioactive, biochemical, and physicochemical properties of the Dolichos lablab bean. Food Funct. 2017, 8, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Ahmed, R.; Bhowmick, S.; Mamun, A.A.; Hashimoto, M. Proximate composition and fatty acid analysis of Lablab purpureus (L.) legume seed: Implicates to both protein and essential fatty acid supplementation. Springerplus 2016, 5, 1899. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-C. Chinese Medicinal Herbs: A Modern Edition of a Classic Sixteenth-Century Manual; Dover Publications: Mineola, NY, USA, 2003. [Google Scholar]
- Oh, S.-W.; Ryu, B.-H. Experimental Studies on the Antidiarrheal Effects of Anjang-san. JIKM 2011, 32, 54–66. [Google Scholar]
- Kichu, M.; Malewska, T.; Akter, K.; Imchen, I.; Harrington, D.; Kohen, J.; Vemulpad, S.R.; Jamie, J.F. An ethnobotanical study of medicinal plants of Chungtia village, Nagaland, India. J. Ethnopharmacol. 2015, 166, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, S.V.; Gebhart, G.F. A role for spinal nitric oxide in mediating visceral hyperalgesia in the rat. Gastroenterology 1999, 116, 1399–1408. [Google Scholar] [CrossRef]
- Park, B.K.; Chun, E.; Choi, J.J.; Shin, Y.; Kho, Y.T.; Oh, S.H.; Kim, S.Y.; Lee, T.H.; Kim, T.W.; Shin, E.; et al. Administration of Wasabia koreana Ameliorates Irritable Bowel Syndrome-Like Symptoms in a Zymosan-Induced Mouse Model. J. Med. Food 2017, 20, 474–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Zou, N.; Li, J.; Lv, H.; Wei, J.; Fang, X.C.; Qian, J.M. Elevated expression of c-fos in central nervous system correlates with visceral hypersensitivity in irritable bowel syndrome (IBS): A new target for IBS treatment. Int. J. Colorectal Dis. 2011, 26, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Liu, S.B.; Chen, T.; Koga, K.; Zhang, T.; Li, Y.Q.; Zhuo, M. Effects of NB001 and gabapentin on irritable bowel syndrome-induced behavioral anxiety and spontaneous pain. Mol. Brain 2014, 7, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimball, E.S.; Palmer, J.M.; D’Andrea, M.R.; Hornby, P.J.; Wade, P.R. Acute colitis induction by oil of mustard results in later development of an IBS-like accelerated upper GI transit in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G1266–G1273. [Google Scholar] [CrossRef] [PubMed]
- Laird, J.M.; Martinez-Caro, L.; Garcia-Nicas, E.; Cervero, F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain 2001, 92, 335–342. [Google Scholar] [CrossRef]
- Morgan, V.; Pickens, D.; Gautam, S.; Kessler, R.; Mertz, H. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut 2005, 54, 601–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pruzanski, W.; Stefanski, E.; Vadas, P.; Ramamurthy, N.S. Inhibition of extracellular release of proinflammatory secretory phospholipase A2 (sPLA2) by sulfasalazine: A novel mechanism of anti-inflammatory activity. Biochem. Pharmacol. 1997, 53, 1901–1907. [Google Scholar] [CrossRef]
- Gwee, K.A. Post-Infectious Irritable Bowel Syndrome, an Inflammation-Immunological Model with Relevance for Other IBS and Functional Dyspepsia. J. Neurogastroenterol. Motil. 2010, 16, 30–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.C. Psychological Co-morbidity in Functional Gastrointestinal Disorders: Epidemiology, Mechanisms and Management. J. Neurogastroenterol. Motil. 2012, 18, 13–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.T.; Hu, L.Y.; Shen, C.C.; Huang, M.W.; Tsai, S.J.; Yang, A.C.; Hu, C.K.; Perng, C.L.; Huang, Y.S.; Hung, J.H. Risk of Psychiatric Disorders following Irritable Bowel Syndrome: A Nationwide Population-Based Cohort Study. PLoS ONE 2015, 10, e0133283. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.Y.; Schaukowitch, K.; Farbiak, L.; Kilaru, G.; Kim, T.K. Stimulus-specific combinatorial functionality of neuronal c-fos enhancers. Nat. Neurosci. 2016, 19, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Clement, C.I.; Keay, K.A.; Owler, B.K.; Bandler, R. Common patterns of increased and decreased fos expression in midbrain and pons evoked by noxious deep somatic and noxious visceral manipulations in the rat. J. Comp. Neurol. 1996, 366, 495–515. [Google Scholar] [CrossRef]
- Yokota, T.; Baba, J.; Takahashi, N. Brassinolide-related Bioactive Sterols in Dolichos lablab: Brassinolide, Castasterone and a New Analog, Homodolicholide. Agric. Biol. Chem. 1983, 47, 1409–1411. [Google Scholar] [CrossRef]
- Fujihara, S.; Nakashima, T.; Kurogochi, Y.; Yamaguchi, M. Distribution and Metabolism of sym-Homospermidine and Canavalmine in the Sword Bean Canavalia gladiata cv Shironata. Plant Physiol. 1986, 82, 795–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamadi, N.; Sharififar, F.; Pournamdari, M.; Ansari, M. A Review on Biosynthesis, Analytical Techniques, and Pharmacological Activities of Trigonelline as a Plant Alkaloid. J. Diets Suppl. 2017, 15, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Chau, C.F.; Cheung, P.C.K.; Wong, Y.S. Effects of cooking on content of amino acids and antinutrients in three Chinese indigenous legume seeds. J Sci Food Agric 1999, 75, 447–452. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Hu, C.A. Therapeutic Potential of Amino Acids in Inflammatory Bowel Disease. Nutrients 2017, 9, 920. [Google Scholar] [CrossRef] [PubMed]








| Group | Length Score | Weight Score | Stool Score | Macroscopic Score |
|---|---|---|---|---|
| Naïve | 0.20 ± 0.40 | 0.00 ± 0.00 | 0.60 ± 0.58 | 0.80 ± 0.86 |
| Control | 1.40 ± 0.50 ### | 3.25 ± 0.50 ### | 2.95 ± 0.84 ### | 6.95 ± 1.72 ### |
| DL100 | 0.00 ± 0.00 *** | 1.25 ± 0.96 | 1.30 ± 0.69 ** | 2.30 ± 1.58 *** |
| DL200 | 0.00 ± 0.00 *** | 1.00 ± 0.82 * | 1.00 ± 0.50 ** | 1.80 ± 1.04 *** |
| DL400 | 0.00 ± 0.00 *** | 1.25 ± 0.96 | 1.25 ± 0.73 ** | 2.25 ± 1.24 *** |
| AMT | 0.00 ± 0.00 *** | 2.25 ± 0.96 | 1.45 ± 0.82 * | 3.25 ± 1.79 ** |
| SSZ | 0.40 ± 0.50 ** | 1.00 ± 0.82 * | 1.45 ± 0.41 * | 2.65 ± 1.42 *** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun, E.; Yoon, S.; Parveen, A.; Jin, M. Alleviation of Irritable Bowel Syndrome-Like Symptoms and Control of Gut and Brain Responses with Oral Administration of Dolichos lablab L. in a Mouse Model. Nutrients 2018, 10, 1475. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10101475
Chun E, Yoon S, Parveen A, Jin M. Alleviation of Irritable Bowel Syndrome-Like Symptoms and Control of Gut and Brain Responses with Oral Administration of Dolichos lablab L. in a Mouse Model. Nutrients. 2018; 10(10):1475. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10101475
Chicago/Turabian StyleChun, Eunho, Soojung Yoon, Amna Parveen, and Mirim Jin. 2018. "Alleviation of Irritable Bowel Syndrome-Like Symptoms and Control of Gut and Brain Responses with Oral Administration of Dolichos lablab L. in a Mouse Model" Nutrients 10, no. 10: 1475. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10101475