Renoprotective Effects of Antroquinonol in Rats with Nω-Nitro-l-Arginine Methyl Ester-Induced Hypertension
Abstract
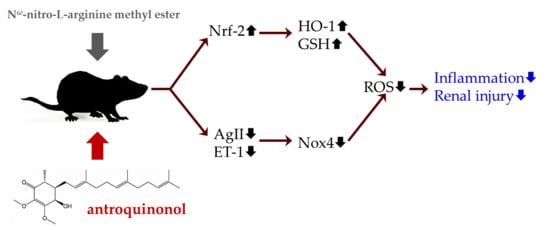
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Blood Pressure Measurement
2.3. Blood Collection and Analysis
2.4. Renal Tissue Collection and Analysis
2.5. Statistical Analysis
3. Results
3.1. Body Weight, Food Intake, and Blood Pressure
3.2. Endothelial Dysfunction and Kidney Injury
3.3. Oxidative Stress
3.4. Inflammatory Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| NO | nitric oxide |
| Ang | angiotensin |
| ET | endothelium |
| NOX | NADPH oxidase |
| Nrf | nuclear factor erythroid |
| HO | heme oxygenase |
| TNF | tumor necrosis factor |
| IL | interleukin |
| ALT | alanine aminotransferase; |
| AST | aspartate aminotransferase |
| PAI | plasminogen activator inhibitor |
| MDA | malondialdehyde |
| NF-κB | nuclear factor-κB |
| IκB | inhibitor of NF-κB |
References
- Brandes, R.P. Endothelial dysfunction and hypertension. Hypertension 2014, 64, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Bourque, S.L.; Davidge, S.T.; Adams, M.A. The interaction between endothelin-1 and nitric oxide in the vasculature: New perspectives. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R1288–R1295. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, A.; Nasri, H.; Rafieian-Kopaei, M. Oxidative stress and hypertension: Possibility of hypertension therapy with antioxidants. J. Res. Med. Sci. 2014, 19, 358–367. [Google Scholar] [PubMed]
- Montezano, A.C.; Dulak-Lis, M.; Tsiropoulou, S.; Harvey, A.; Briones, A.M.; Touyz, R.M. Oxidative stress and human hypertension: Vascular mechanisms, biomarkers, and novel therapies. Can. J. Cardiol. 2015, 31, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Pullikotil, P.; Chen, H.; Muniyappa, R.; Greenberg, C.C.; Yang, S.; Reiter, C.E.; Lee, J.W.; Chung, J.H.; Quon, M.J. Epigallocatechin gallate induces expression of heme oxygenase-1 in endothelial cells via p38 MAPK and Nrf-2 that suppresses proinflammatory actions of TNF-alpha. J. Nutr. Biochem. 2012, 23, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Geethangili, M.; Tzeng, Y.M. Review of pharmacological effects of antrodia camphorata and its bioactive compounds. Evid. Based Comp. Altern. Med. 2011, 2011, 17. [Google Scholar]
- Yang, S.S.; Wang, G.J.; Wang, S.Y.; Lin, Y.Y.; Kuo, Y.H.; Lee, T.H. New constituents with iNOS inhibitory activity from mycelium of Antrodia camphorata. Planta Med. 2009, 75, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.M.; Lee, Y.R.; Hung, L.M.; Liu, S.Y.; Kuo, M.T.; Wen, W.C.; Chen, P. An extract of antrodia camphorata mycelia attenuates the progression of nephritis in systemic lupus erythematosus-prone NZB/W. F1 mice. Evid. Based Comp. Altern. Med. 2011, 2011, 1–7. [Google Scholar]
- Tsai, P.Y.; Ka, S.M.; Chao, T.K.; Chang, J.M.; Lin, S.H.; Li, C.Y.; Kuo, M.T.; Chen, P.; Chen, A. Antroquinonol reduces oxidative stress by enhancing the Nrf2 signaling pathway and inhibits inflammation and sclerosis in focal segmental glomerulosclerosis mice. Free Radic. Biol. Med. 2011, 50, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Lee, C.K.; Tsou, W.L.; Liu, S.Y.; Kuo, M.T.; Wen, W.C. A new cytotoxic agent from solid-state fermented mycelium of antrodia camphorata. Planta Med. 2007, 73, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.M.; Ka, S.M.; Hua, K.F.; Wu, T.H.; Chuang, Y.P.; Lin, Y.W.; Yang, F.L.; Wu, S.H.; Yang, S.S.; Lin, S.H.; et al. Antroquinonol mitigates an accelerated and progressive IgA nephropathy model in mice by activating the Nrf2 pathway and inhibiting T cells and NLRP3 inflammasome. Free Radic. Biol. Med. 2013, 61, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Yagi, K. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol. Biol. 1998, 108, 101–106. [Google Scholar] [PubMed]
- Kuandal, A.; Rojas, C.M.; Mysore, K.S. Measurement of NADPH oxidase activity in plants. Bio-Protocol 2012, 2, e278. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Tomita, S.; Ishizawa, K.; Abe, S.; Ikeda, Y.; Kihira, Y.; Tamaki, T. Dietary nitrite ameliorates renal injury in L-NAME-induced hypertensive rats. Nitric Oxide 2010, 22, 98–103. [Google Scholar] [CrossRef] [PubMed]
- D’Amours, M.; Lebel, M.; Grose, J.H.; Lariviere, R. Renal and vascular effects of chronic nitric oxide synthase inhibition: Involvement of endothelin 1 and angiotensin II. Can. J. Physiol. Pharmacol. 1999, 77, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Mayet, J.; Hughes, A. Cardiac and vascular pathophysiology in hypertension. Heart 2003, 89, 1104–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Redondo, A.B.; Aguado, A.; Briones, A.M.; Salaices, M. NADPH oxidases and vascular remodeling in cardiovascular diseases. Pharmacol. Res. 2016, 114, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Sedeek, M.; Nasrallah, R.; Touyz, R.M.; Hebert, R.L. NADPH oxidases, reactive oxygen species, and the kidney: Friend and foe. J. Am. Soc. Nephrol. 2013, 24, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Sachse, A.; Wolf, G. Angiotensin II-induced reactive oxygen species and the kidney. J. Am. Soc. Nephrol. 2007, 18, 2439–2446. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Watts, S.W.; Banes, A.K.; Galligan, J.J.; Fink, G.D.; Chen, A.F. NADPH oxidase-derived superoxide augments endothelin-1-induced venoconstriction in mineralocorticoid hypertension. Hypertension 2003, 42, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Howden, R. Nrf2 and cardiovascular defense. Oxid. Med. Cell Longev. 2013, 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Javkhedkar, A.A.; Quiroz, Y.; Rodriguez-Iturbe, B.; Vaziri, N.D.; Lokhandwala, M.F.; Banday, A.A. Resveratrol restored Nrf2 function, reduced renal inflammation, and mitigated hypertension in spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R840–R846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndisang, J.F. Role of heme oxygenase in inflammation, insulin-signalling, diabetes and obesity. Mediat. Inflamm. 2010, 2010, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ndisang, J.F.; Chibbar, R. Heme oxygenase improves renal function by potentiating podocyte-associated proteins in Nω-Nitro-l-Arginine-Methyl Ester (l-NAME)-Induced hypertension. Am. J. Hypertens. 2015, 28, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Wang, X.Q.; Oveisi, F.; Rad, B. Induction of oxidative stress by glutathione depletion causes severe hypertension in normal rats. Hypertension 2000, 36, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Khattab, M.M.; Nagi, M.N. Thymoquinone supplementation attenuates hypertension and renal damage in nitric oxide deficient hypertensive rats. Phytot. Res. 2007, 21, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.J.; Chu, F.H.; Hsieh, H.W.; Liao, J.W.; Li, W.H.; Lin, J.C.; Shaw, J.F.; Wang, S.Y. Antroquinonol from ethanolic extract of mycelium of Antrodia cinnamomea protects hepatic cells from ethanol-induced oxidative stress through Nrf-2 activation. J. Ethnopharmacol. 2011, 136, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Iturbe, B.; Quiroz, Y.; Ferrebuz, A.; Parra, G.; Vaziri, N.D. Evolution of renal interstitial inflammation and NF-kappaB activation in spontaneously hypertensive rats. Am. J. Nephrol. 2004, 24, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Christian, F.; Smith, E.L.; Carmody, R.J. The Regulation of NF-kappaB Subunits by Phosphorylation. Cells 2016, 5, E12. [Google Scholar] [CrossRef] [PubMed]
- Udwan, K.; Brideau, G.; Fila, M.; Edwards, A.; Vogt, B.; Doucet, A. Oxidative stress and nuclear factor kappaB (NF-kappaB) increase peritoneal filtration and contribute to ascites formation in nephrotic syndrome. J. Biol. Chem. 2016, 291, 11105–11113. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.W.; Pahl, M.V.; Vaziri, N.D. Spontaneous leukocyte activation and oxygen-free radical generation in end-stage renal disease. Kidney Int. 2007, 71, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarajan, V.; Tsai, M.J.; Weng, C.F. Antroquinonol targets FAK-Signaling pathway suppressed cell migration, invasion, and tumor growth of C6 glioma. PLoS ONE 2015, 10, e0141285. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Ho, C.L.; Kao, W.Y.; Chen, Y.M. A phase I multicenter study of antroquinonol in patients with metastatic non-small-cell lung cancer who have received at least two prior systemic treatment regimens, including one platinum-based chemotherapy regimen. Mol. Clin. Oncol. 2015, 3, 1375–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riyaphan, J.; Jhong, C.H.; Lin, S.R.; Chang, C.H.; Tsai, M.J.; Lee, D.N.; Sung, P.J.; Leong, M.K.; Weng, C.F. Hypoglycemic efficacy of docking selected natural compounds against α-Glucosidase and α-Amylase. Molecules 2018, 23, 2260. [Google Scholar] [CrossRef] [PubMed]






| C | H | A | B | |
|---|---|---|---|---|
| Serum | ||||
| ALB (g/L) | 37.40 ± 0.56 | 36.70 ± 0.60 | 36.20 ± 1.02 | 36.40 ± 0.65 |
| BUN (mg/dL) | 14.60 ± 0.50 | 15.80 ± 0.79 | 15.50 ± 0.52 | 15.20 ± 0.59 |
| Cr (mg/dL) | 0.29 ± 0.02 b | 0.38 ± 0.04 a | 0.29 ± 0.02 b | 0.29 ± 0.02 b |
| AST (IU/L) | 73.00 ± 2.10 | 81.80 ± 4.42 | 77.50 ± 4.12 | 74.80 ± 3.59 |
| ALT (IU/L) | 34.80 ± 1.60 b | 50.50 ± 4.17 a | 46.60 ± 2.85 a | 35.30 ± 2.77 b |
| PAI-1 (ng/ml) | 0.35 ± 0.03 c | 0.66 ± 0.09 a | 0.53 ± 0.04 ab | 0.44 ± 0.07 bc |
| ET-1 (pg/mL) | 0.95 ± 0.06 b | 1.19 ± 0.10 a | 0.97 ± 0.10 ab | 0.89 ± 0.05 b |
| AngII (pg/mL) | 358.3 ± 29.9 ab | 402.7 ± 65.2 a | 332.2 ± 31.6 b | 310.9 ± 22.6 b |
| MDA (μmol/L) | 11.76 ± 0.60 b | 16.05 ± 1.81 a | 12.71 ± 0.57 b | 12.05 ± 0.50 b |
| Kidneys | ||||
| ET-1 (pg/mg protein) | 1.2 ± 0.1 b | 1.4 ± 0.1 a | 1.0 ± 0.1 bc | 0.9 ± 0.1 c |
| MDA (nmol/mg protein) | 2.3 ± 0.1 ab | 2.5 ± 0.1 a | 2.1 ± 0.1 bc | 1.8 ± 0.1 c |
| FRAP (nmol/mg protein) | 59.0 ± 4.0 b | 38.7 ± 3.8 c | 59.1 ± 4.2 b | 95.4 ± 10.4 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-R.; Ko, J.; Yeh, W.-J.; Huang, W.-C.; Yang, H.-Y. Renoprotective Effects of Antroquinonol in Rats with Nω-Nitro-l-Arginine Methyl Ester-Induced Hypertension. Nutrients 2018, 10, 1521. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10101521
Chen J-R, Ko J, Yeh W-J, Huang W-C, Yang H-Y. Renoprotective Effects of Antroquinonol in Rats with Nω-Nitro-l-Arginine Methyl Ester-Induced Hypertension. Nutrients. 2018; 10(10):1521. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10101521
Chicago/Turabian StyleChen, Jiun-Rong, Jung Ko, Wan-Ju Yeh, Wen-Chih Huang, and Hsin-Yi Yang. 2018. "Renoprotective Effects of Antroquinonol in Rats with Nω-Nitro-l-Arginine Methyl Ester-Induced Hypertension" Nutrients 10, no. 10: 1521. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10101521